1
MIGRAINE MANAGEMENT
Migraine management should involve both acute and preventive treatments.
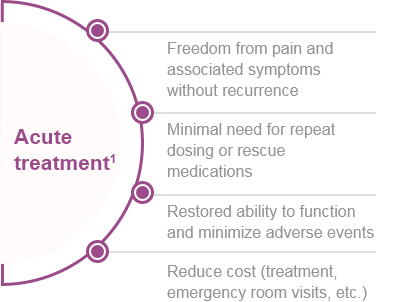
Acute/Abortive Treatment1,2
Goals:
- Rapid and consistent freedom from pain and associated symptoms without recurrence1,2
- Restored ability to function1,2
- Minimal need for repeat dosing or rescue medications1,2
- Optimal self-care and reduced subsequent use of resources (e.g., emergency room visits, diagnostic imaging, clinician, and ambulatory infusion center visits)1
- Minimal or no adverse events1
- Cost consideration1
Preventive Treatment1
Goals:
- Reduce attack frequency, severity, duration, and disability
- Improve responsiveness to and avoid escalation in use of acute treatment
- Improve function and reduce disability
- Reduce reliance on poorly tolerated, ineffective, or unwanted acute treatments
- Reduce costs associated with migraine treatment
- Enable patients to manage their own disease
- Improve health-related quality of life
- Reduce headache-related distress and psychological symptoms
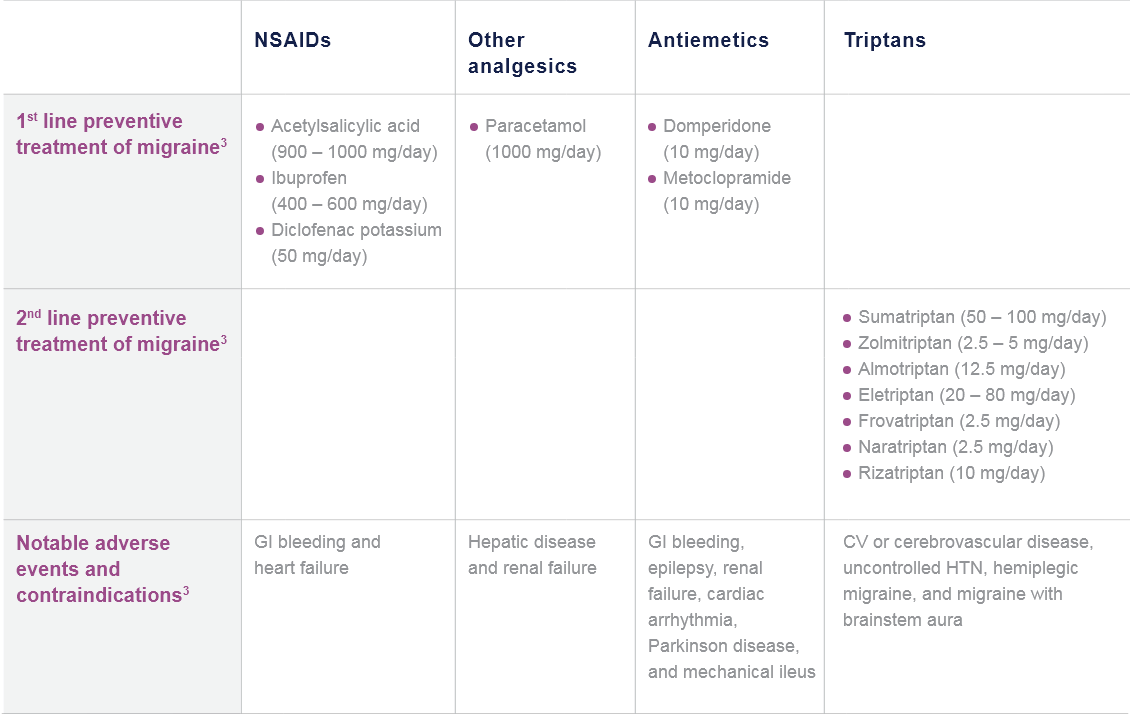
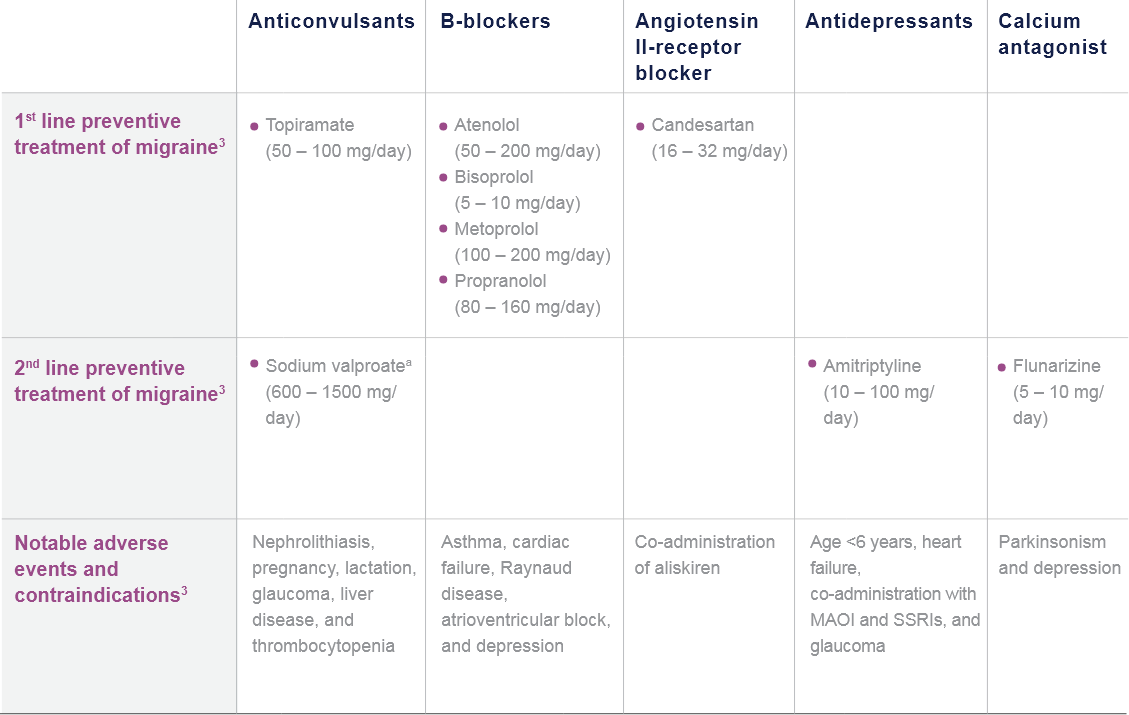
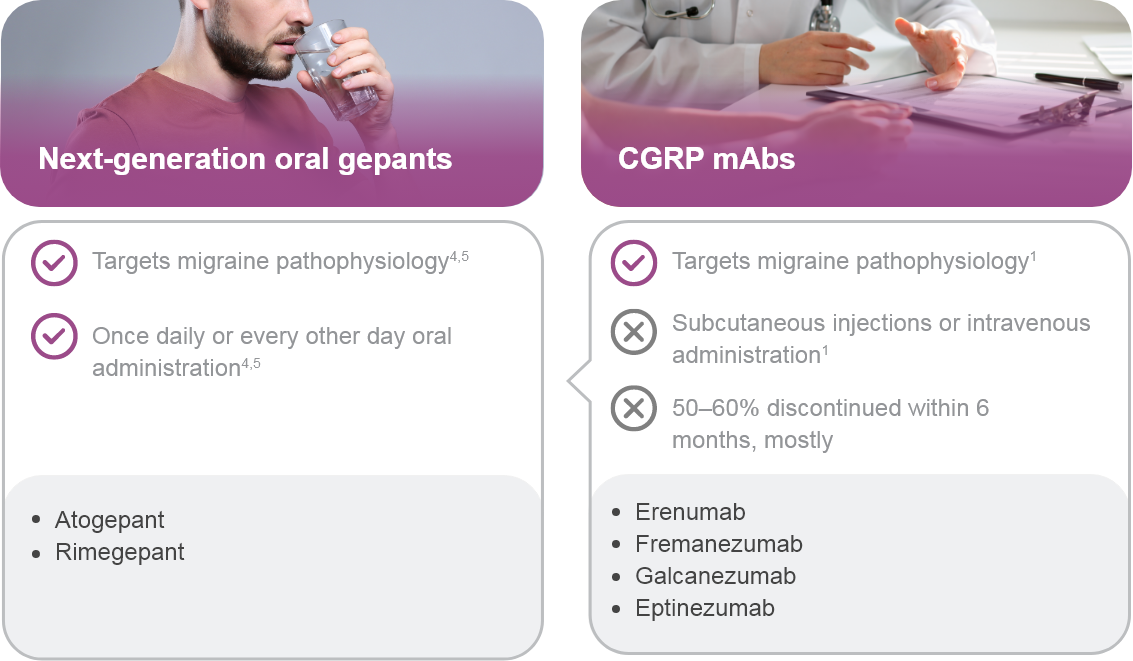
CGRP: Calcitonin gene-related peptide; mAb: Monoclonal antibody; NSAID: Non-steroidal anti-inflammatory drug.
GUIDELINES
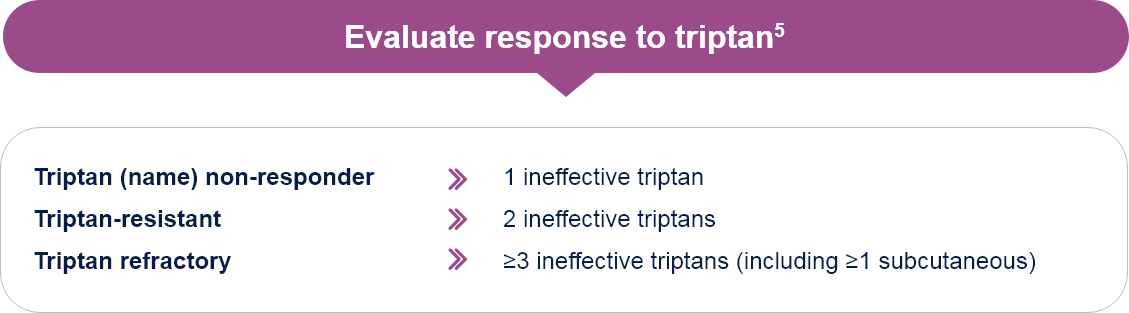
EHF Consensus:
Effective Treatment of Migraine Attack and Triptan Response
Reaching, within 2 hours from intake of the drug, and maintaining, for ≥24 hours a well-being status as defined by the following:5
- Improvement of headache from severe or moderate to mild or absent
- Absent or minimal disturbances due to migraine-related non-pain symptoms
- No meaningful drug-related adverse events
Effective attack treatment in ≥3 out of 4 consecutive attacks
EHF: European Headache Federation.
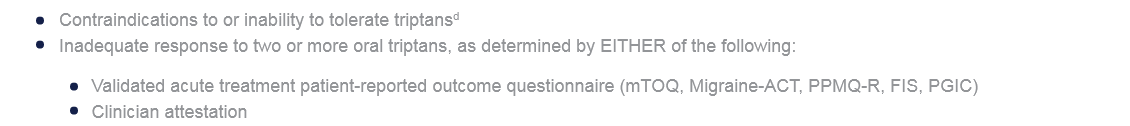
Use is appropriate when ALL the following are met:1
- Prescribed/recommended by a licensed clinician
- Patient is at least 18 years of agec
- Diagnosis of ICHD-3 migraine with aura, migraine without aura, or chronic migraine
- Either of the following:
aConsider neuromodulatory devices in patients who prefer non-drug treatments or in whom drug treatment is ineffective, intolerable, or contraindicated.1
bTo improve the likelihood of choosing appropriate therapy at the initial consultation and adjust these recommendations to the needs of individual patients.1
cThree neuromodulatory devices (nVNS, REN, and sTMS) have also received clearance for the treatment of patients aged 12–17 years.1
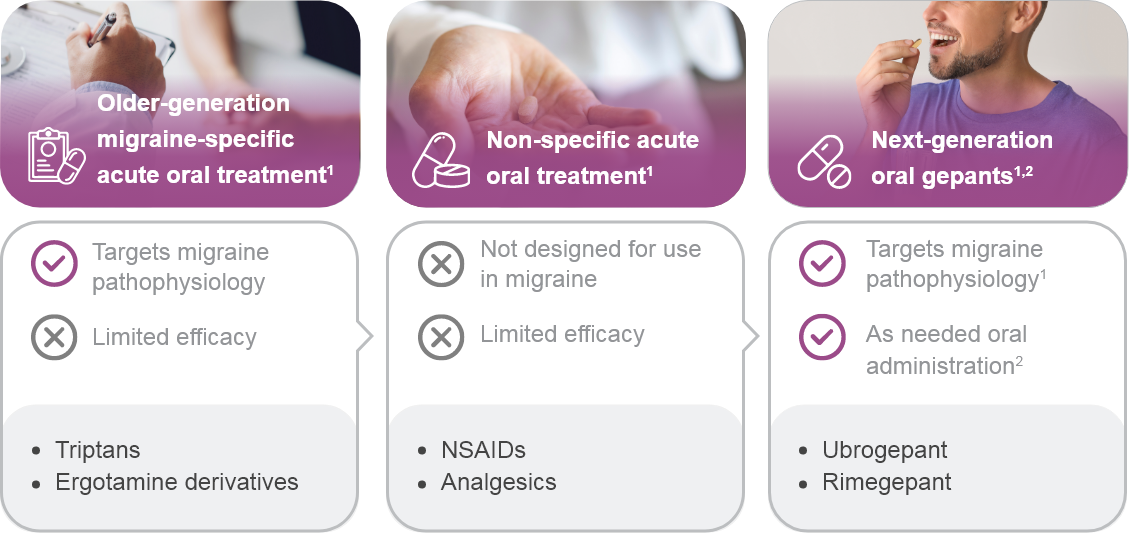
dGepants, ditans, and neuromodulatory devices may be considered.1
FIS: Functional Impairment Scale; ICHD-3: International Classification of Headache Disorders, 3rd edition; Migraine-ACT: Migraine Assessment of Current Therapy; mTOQ: Migraine Treatment Optimization Questionnaire; NSAID: Non-steroidal anti-inflammatory drug;
PGIC: Patient Global Impression of Change; PPMQ-R: Patient Perception of Migraine Questionnaire-Revised.
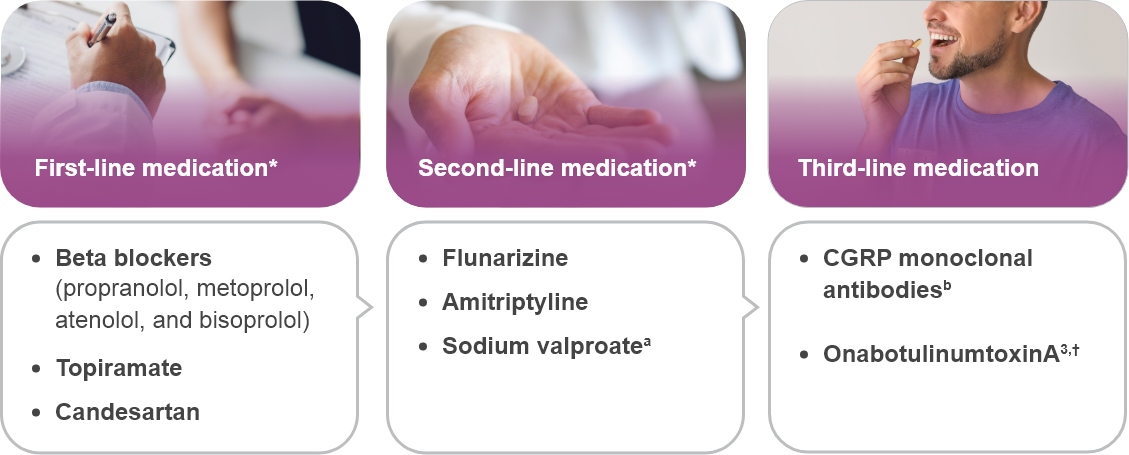
*Traditional treatment.
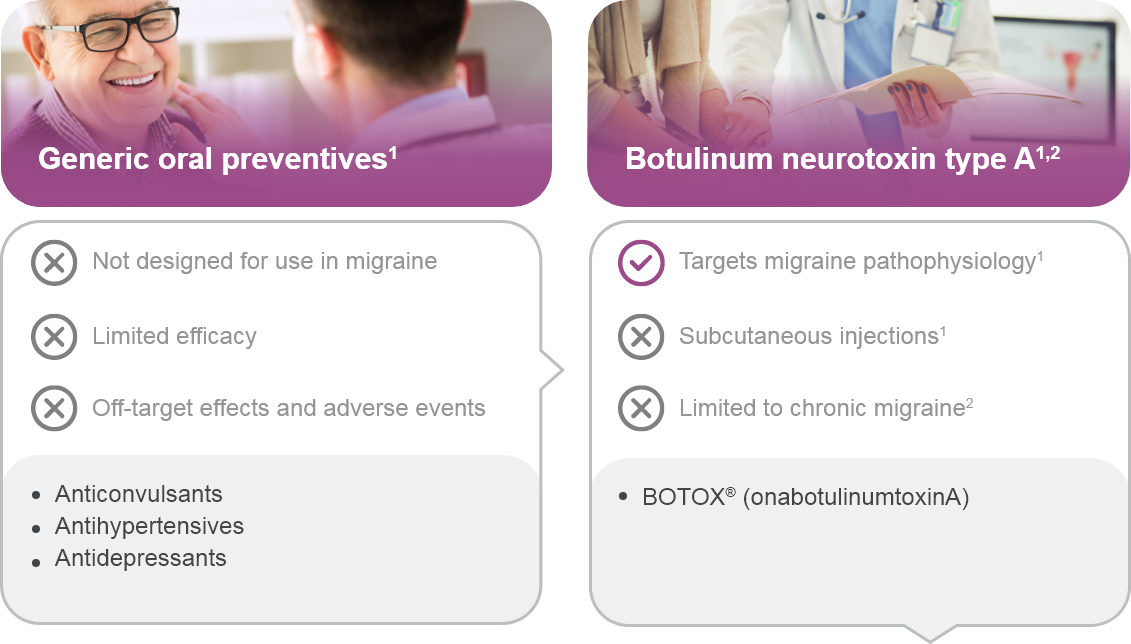
†Limited to chronic migraine only.
aSodium valproate is absolutely contraindicated in women of childbearing potential.
bCGRP monoclonal antibodies target CGRP or its receptor.
cPrevention of chronic migraine.
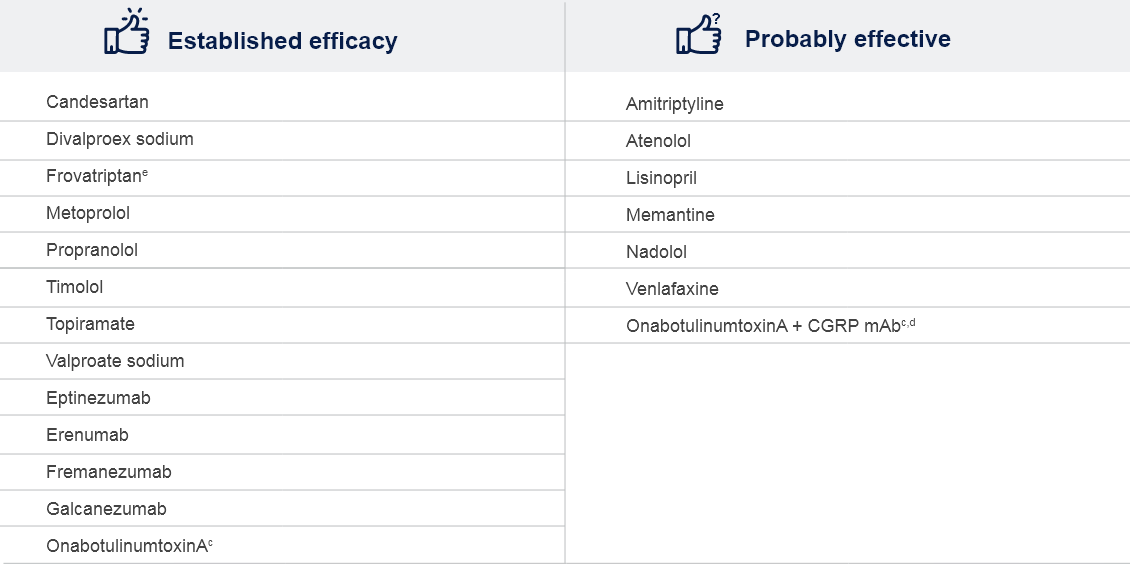
dOne Class IV trial based on AAN evidence classification.
eShort-term prevention of menstrual-related migraine; evaluated and rejected by the FDA for this indication.
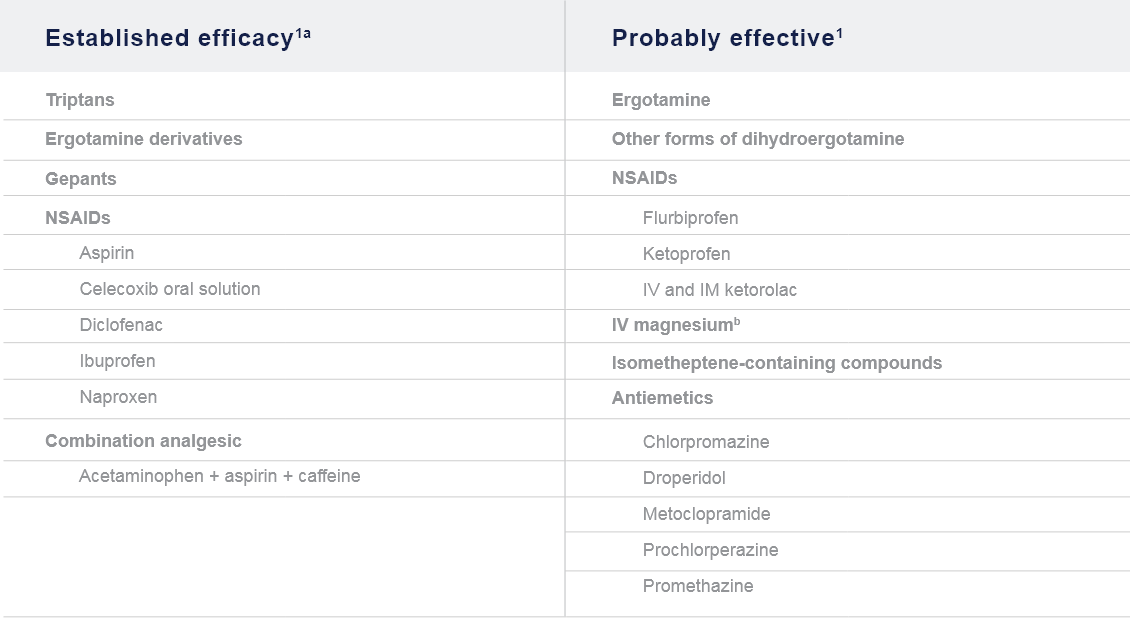
AAN: American Academy of Neurology; CGRP: Calcitonin gene-receptor peptide; EAN: European Academy of Neurology; EHF: European Headache Federation; FDA: Food and Drug Administration; mAb: Monoclonal antibody; NSAID: Non-steroidal anti-inflammatory drug;
OnabotulinumtoxinA: Botulinum toxin type A.
References
1. Ailani J, et al. Headache. 2021;61(7):1021-1039.
2. Tzankova V, et al. CMAJ. 2023;195(4):E153-E158.
3. Eigenbrodt AK, et al. Nat Rev Neurol. 2021;17(8):501-514.
4. Worthington I, et al. Can J Neurol Sci. 2013;405(Suppl 3):S1-S80.
5. Sacco S, et al. J Headache Pain. 2022;23(1):133.
6. Bendtsen L, et al. J Headache Pain. 2018;19(1):91.
7. Headache Classification Committee of the International Headache Society (IHS). Cephalalgia 2018;38:1–211.
8. QULIPTA [package insert]. Dublin, Ireland: AbbVie. October 2021.
9. UBRELVY [package insert]. Madison, NJ: Allergan USA, Inc. March 2021.
10. NURTEC [package insert]. New Haven, CT: Biohaven. April 2022.