This promotional website is for UK Healthcare Professionals involved in the management of haematological malignancies. Adverse event reporting information can be found below.
Treatment-experienced CLL
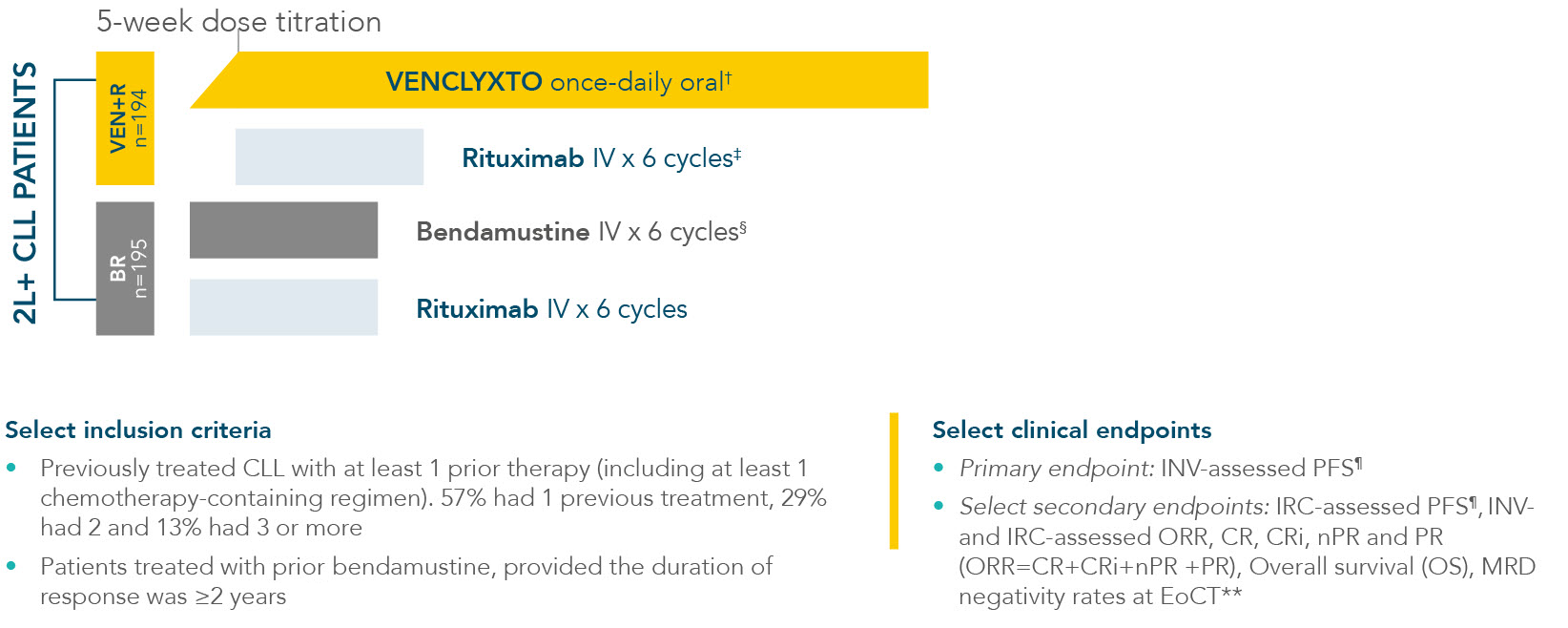
MURANO: designed to allow patients to complete treatment in 2 years, following a 5-week dose titration1,2*
MURANO evaluated VENCLYXTO + rituximab vs a standard CIT regimen (bendamustine + rituximab)1
*MURANO was a multicentre, open-label, randomised Phase III trial to evaluate the benefit of Venetoclax plus Rituximab compared with Bendamustine plus Rituximab in relapsed/refractory patients with CLL. Treatment complete 24 months after cycle 1 day 1 of rituximab.
†After initial 5-week schedule of ramp-up from 20 mg per day VENCLYXTO was administered as 400 mg once daily for 2 years, unless disease progression or unacceptable toxic effects occurred sooner.
‡Rituximab dosing: 375 mg/m2 IV Day 1, Cycle 1 (initiated after the 5-week dose titration schedule); 500 mg/m2 IV Cycles 2-6. Each cycle was 28 days.
§Bendamustine dosing: 70 mg/m2 IV Days 1 and 2, Cycles 1-6. Each cycle was 28 days.
¶Assessed using the iwCLL updated NCI-WG guidelines (2008).
**MRD negativity = <1 tumour cell per 104 white cells. MRD was evaluated in the peripheral blood and/or bone marrow using ASO-PCR and/or flow cytometry.
Rate of IRC-assessed CR† at 9 months in the ITT population
(first of the secondary end points to be tested hierarchically)1,2
Complete remission (CR+CRi) rate‡
*Remission is based on CR, ORR and PR. CR is defined in the SmPC as complete remission.
†CR = CR+CRi.
‡The discrepancy between IRC- and INV-assessed CR rates was due to interpretation of residual adenopathy on CT scans.
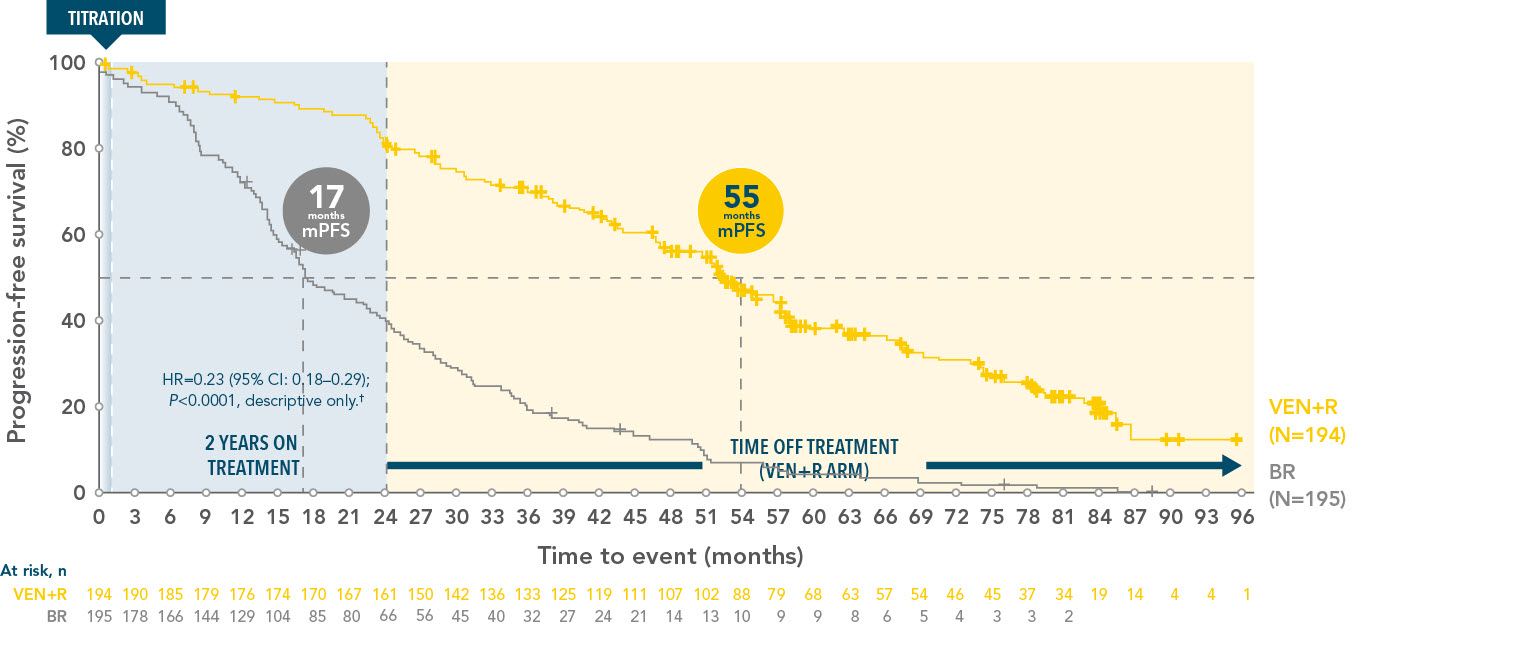
VENCLYXTO+Rituximab demonstrated a longer lasting PFS vs BR at 7 years
PFS (INV-assessed) in the ITT population of VEN+R vs BR at 7 years3*
Adapted from Kater AP et al. 2023.
*In the primary analysis in the ITT population, INV PFS with VEN+R was superior to BR. (0.17; 95% CI 0.11-0.25; P<0.001).1,3
†Stratified HR is presented, unstratified HR=0.25.
Median follow-up was 86.8 months for VEN+R and 84.4 months for BR.
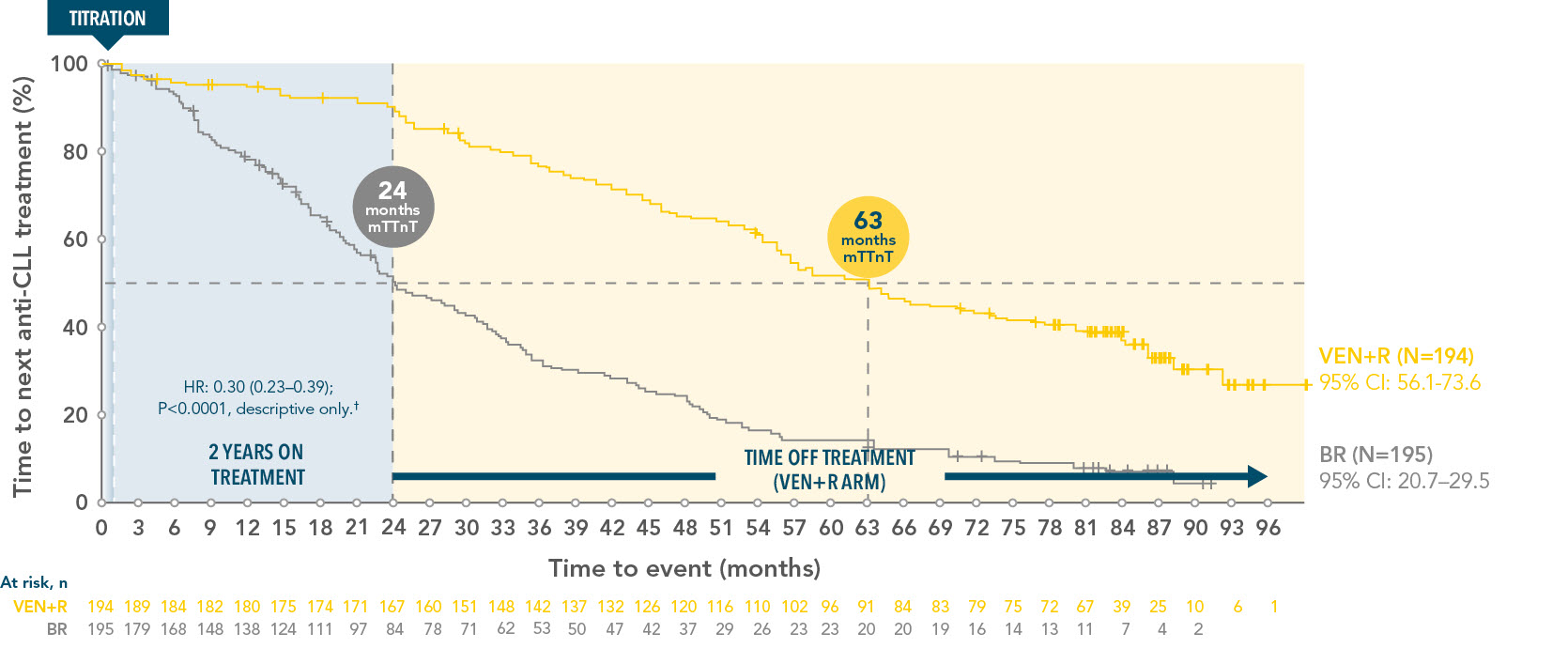
VENCLYXTO+Rituximab demonstrated a median time-to-next-treatment* of 63 months in R/R patients
TTnT in the ITT population of VEN+R vs BR over 7 years3*
Adapted from Kater AP et al. 2023
*TTnT was defined as the time from initiation of BR or VEN+R to next anti-CLL treatment, or death (whichever occurs first).
†Stratified HR is presented, unstratified HR=0.32.
Median follow-up was 86.8 months for VEN+R and 84.4 months for BR.
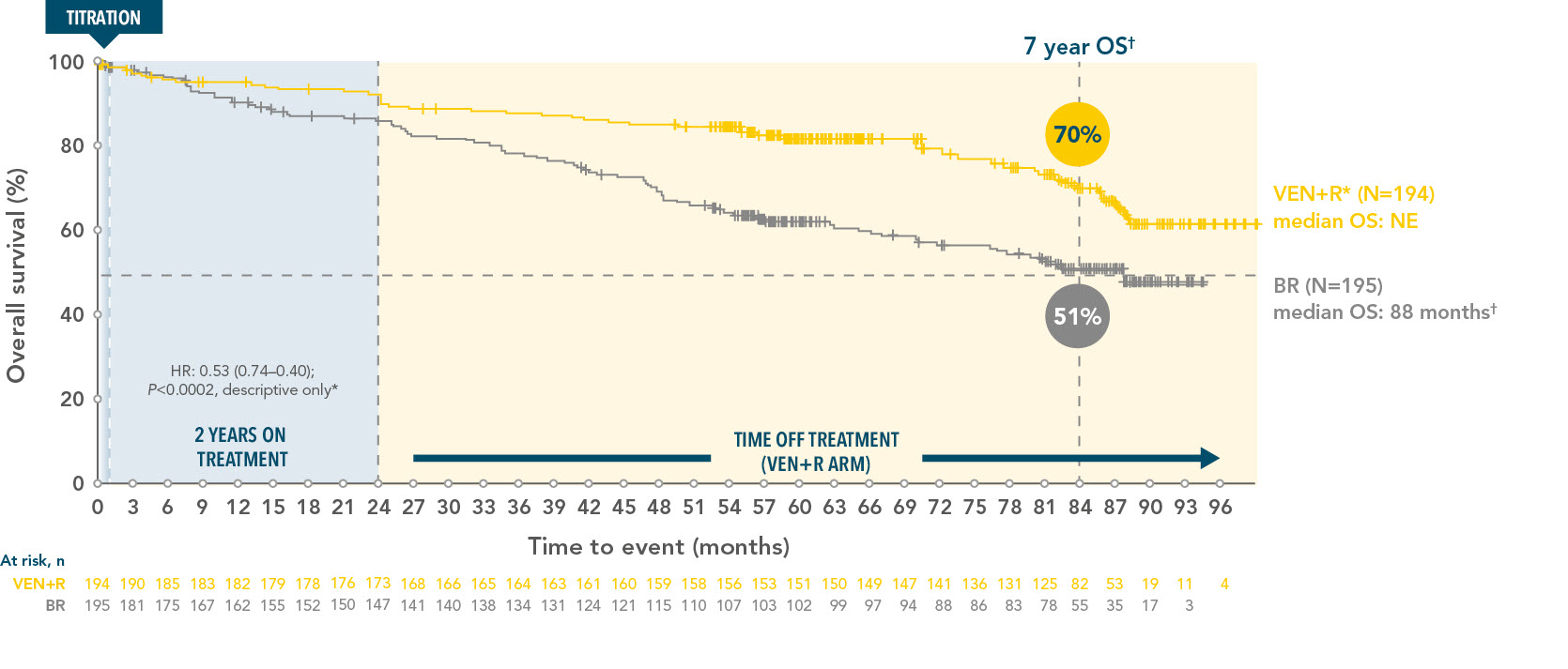
VENCLYXTO+Rituximab demonstrated a longer lasting overall survival vs BR at 7 years
Overall survival in the ITT population at 7 years (secondary endpoint), not tested for statistical significance3
Adapted from Kater AP et al. 2023
*Stratified HR is presented, unstratified HR=0.54.
†Median follow-up was 86.8 months for VEN+R and 84.4 months for BR.
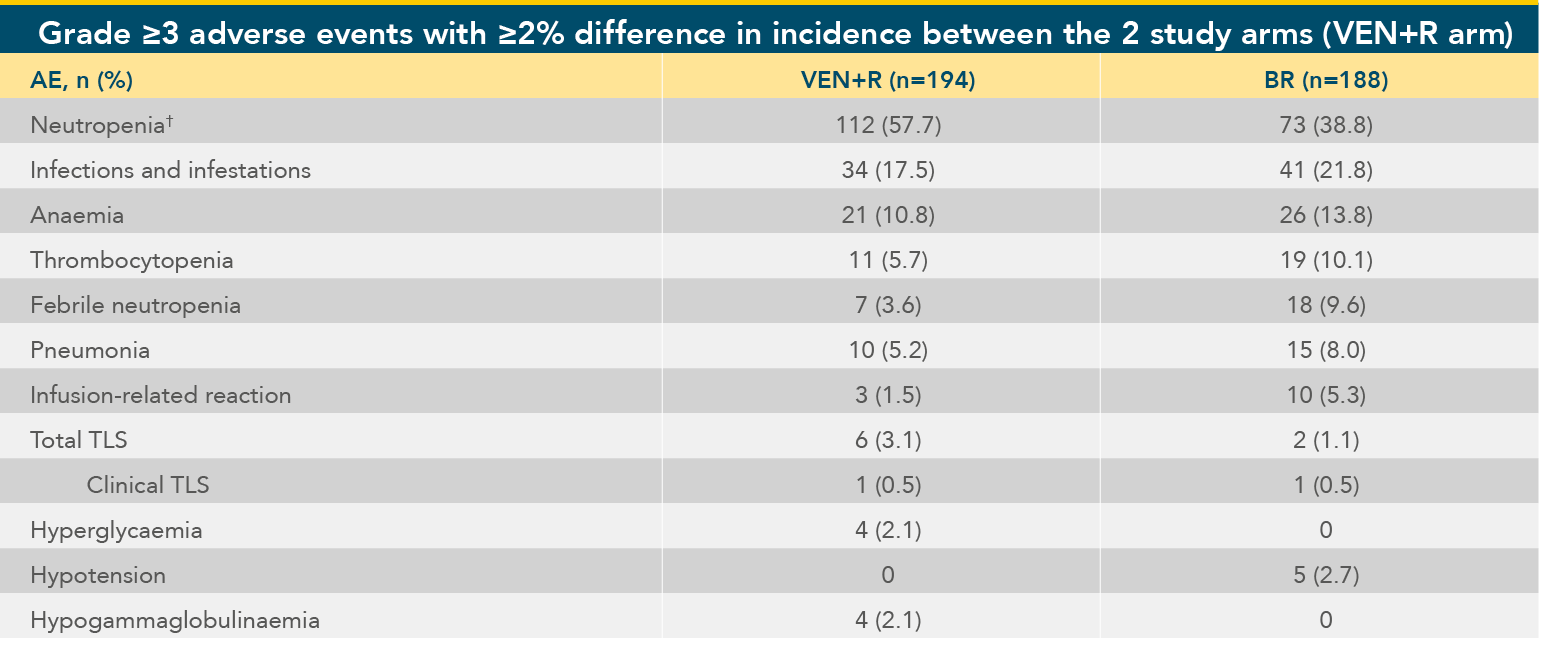
Adverse events in MURANO after median of 22 months VENCLYXTO treatment1*
Grade 3-4 AEs with ≥2% difference in incidence between treatment arms within the safety evaluable population
Adapted from Seymour JF et al. 2018.
*At the time of data cutoff, the median duration of exposure to VENCLYXTO was 22.1 months (0.1-27.9 months).
†A higher percentage of new-onset events of neutropenia occurred during the combination-treatment period
than during the VENCLYXTO monotherapy phase (54.1% vs 11.1%).
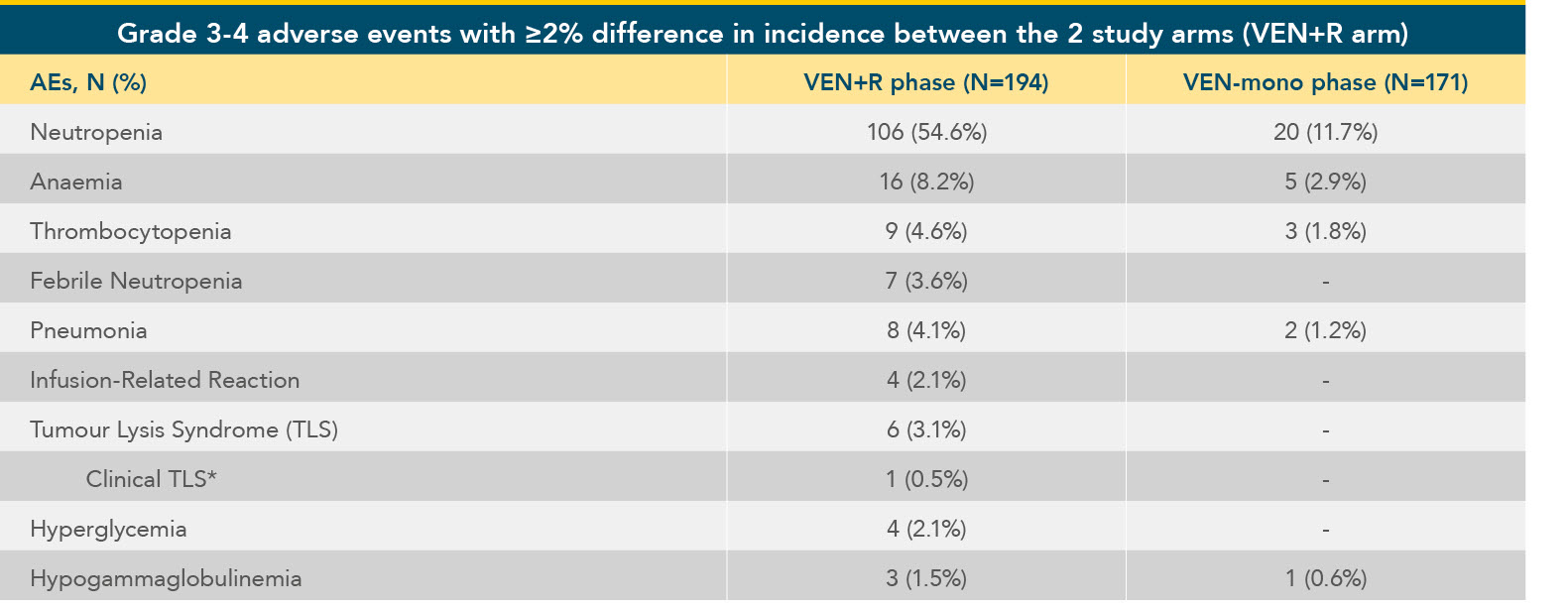
In MURANO, incidence of grade 3 and 4 AEs decreased after the combination treatment period ended4
Haematological toxicities by phase of VEN+R treatment4
Adapted from Seymour JF et al. 2019.
Clinical data cutoff May 8, 2018. Treatment-emergent AEs are included.
*TLS occurred during previous ramp-up schedule prior to current approved treatment ramp-up.
For full safety information, please refer to the SmPC
You might also be interested in
CLL-decision-making in the relapsed/refractory setting
View an expert, case-based panel discussion on the available evidence base for treatment of patients relapsing after CIT, after prior BCRi therapy or after venetoclax-based therapy. Featuring Dr Toby Eyre, Dr Dima El-Sharkawi, Dr Talha Munir, Dr Piers Patten & Dr Stella Williams.
You are advised to read the Prescribing Information and Summary of Product Characteristics to evaluate patient suitability for VENCLYXTO.
VENCLYXTO PRESCRIBING INFORMATION
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
VENCLYXTO SUMMARY OF PRODUCT CHARACTERISTICS
UK-VNCCLL-240485. Date of Preparation: January 2025