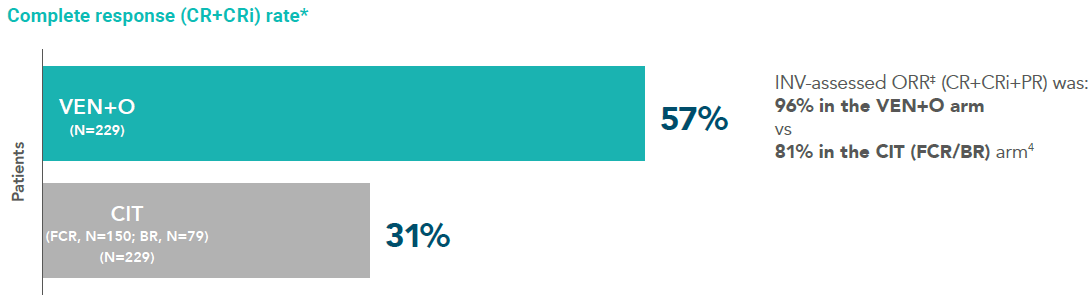This promotional website is for UK Healthcare Professionals involved in the management of haematological malignancies. Adverse event reporting information can be found below.
VENCLYXTO + obinutuzumab in 1st line CLL
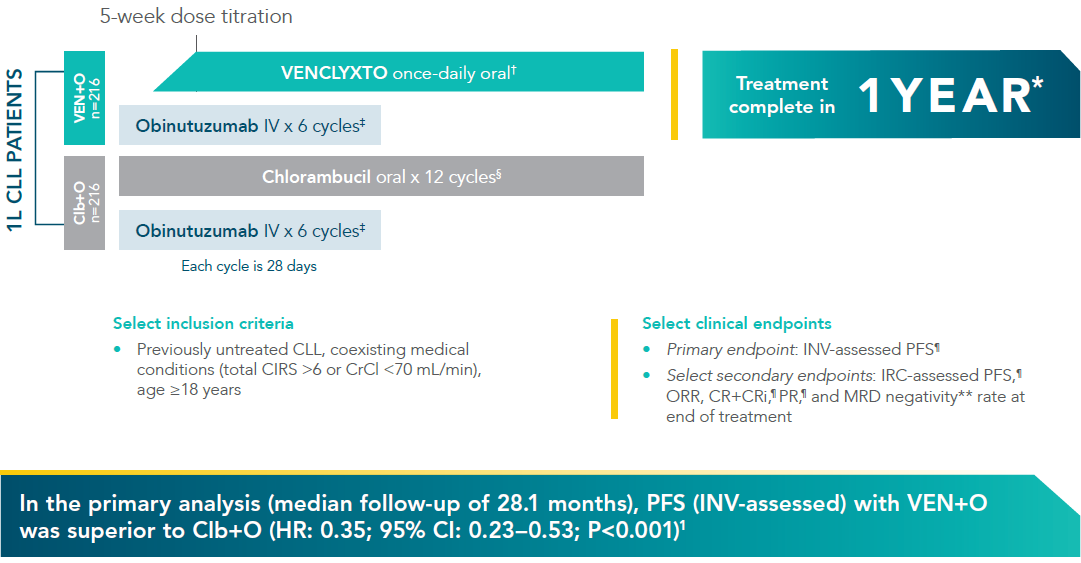
VENCLYXTO + obinutuzumab (VEN+O) vs chlorambucil + obinutuzumab (Clb+O)1,2*
*CLL-14 was a multicentre, open-label, Phase III trial to compare the efficacy and safety of VEN+O Versus Clb+O in previously untreated CLL patients with coexisting medical conditions. Treatment was completed after twelve 28-day cycles.
†VENCLYXTO was started on Cycle 1, Day 22 and increased to full dose for Cycle 3, Day 1. VENCLYXTO was continued until the end of Cycle 12. VENCLYXTO 400 mg daily after initial dose-titration period.
‡Obinutuzumab dosing: 100 mg Cycle 1, Day 1, followed by 900 mg on Days 1 or 2; 1000 mg on Days 8 and 15 and Day 1 of subsequent cycles.
§Chlorambucil dosing: 0.5 mg/kg on Days 1 and 15 of each 28-day cycle.
¶Assessed using the iwCLL updated NCI-WG guidelines (2008).
**MRD negativity was assessed at the end of treatment. MRD negativity = <1 tumour cell per 104 white cells.
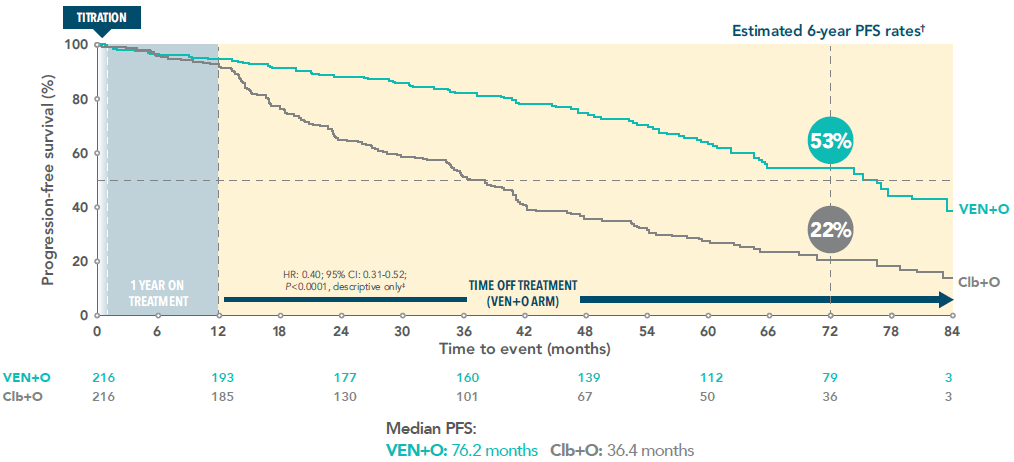
Over twice as many VEN+O patients remained progression-free vs Clb+O 6 years after initiating VEN+O treatment3
PFS (INV-assessed) of VEN+O vs Clb+O at 6 years (primary endpoint)3*
Adapted from Al-Sawaf O et al. 2024.
*In the primary analysis at 28.1 months in the ITT population, PFS with VEN+O was superior to Clb+O (HR: 0.35; 95% CI: 0.23–0.53; P<0.001)1
†Median follow-up 76.4 months.
‡Not tested for statistical significance.
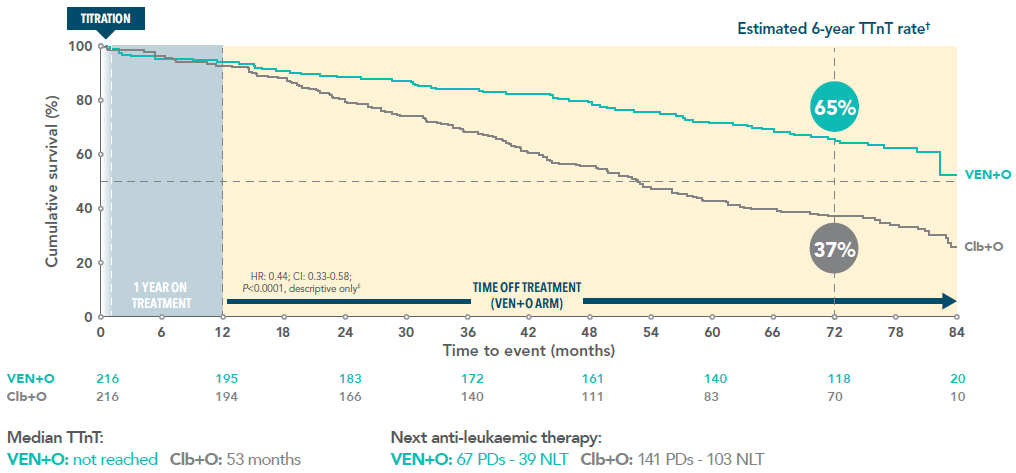
Over 6 out of 10 VEN+O patients were still treatment-free 6 years after initiating treatment3
Time to next treatment* for VEN+O vs Clb+O over 6 years (secondary endpoint)3
Adapted from Al-Sawaf O et al. 2024.
*TTnT was defined as the time between the date of randomisation to the date of first intake of new anti-CLL treatment or death prior to initiating new anti-CLL treatment.
†Median follow-up 76.4 months.
‡Not tested for statistical significance.
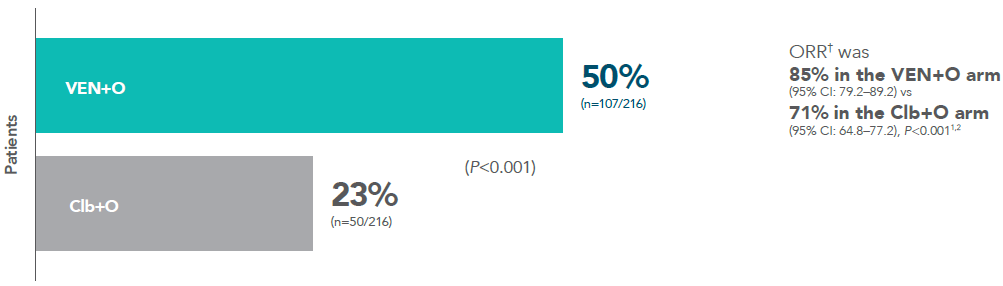
Rate of INV-assessed CR* 3 months after completing treatment (ITT population; secondary endpoint)1
Complete remission (CR+CRi) rate1
Median follow-up time in the primary analysis was 28 months (range: 0-36 months).
*Remission is based on CR and ORR. CR is defined in the SmPC as complete remission.
†ORR=CR+CRi+PR (secondary endpoint).
For full safety information, please refer to the SmPC
CI, Confidence Interval; CIRS, Cumulative illness rating scale; Clb+O, Chlorambucil and Obinutuzumab; CLL, Chronic lymphocytic leukaemia; CrCl, Creatine clearance; CR, Complete response; CRi, CR with incomplete marrow recovery; HR, Hazard ratio; IRC, Independent review committee; INV, Investigator; ITT, Intent-to-treat; IV, Intravenous; iwCLL, International workshop for CLL; MRD, Minimal residual disease; NCI-WG, National Cancer Institute-sponsored working group; NLT, Next anti-leukaemic therapy; ORR, Overall response rate; OS, Overall survival; PD, Disease progression; PFS, Progression-free survival; PR, Partial response; SmPC, Summary of Product Characteristics; TTnT, Time to next treatment; uMRD, Undetectable minimal residual disease; VEN+O, VENCLYXTO + Obinutuzumab; 1L, First line.
References
- Fischer K et al. N Engl J Med. 2019; 380: 2225-36.
- VENCLYXTO Summary of Product Characteristics.
- Al-Sawaf O et al. Blood 2024. 144(18): 1924-1935.
- AI-Sawaf O et al. Abstract S145. EHA Congress 2023.
UK-VNCCLL-250051. Date of Preparation: March 2025
VENCLYXTO + obinutuzumab in 1st line CLL
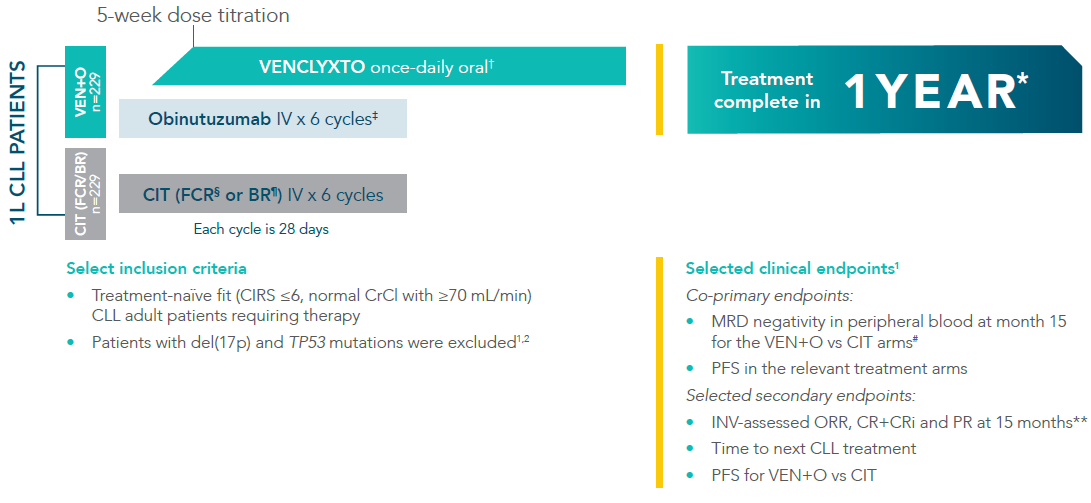
VENCLYXTO + obinutuzumab (VEN+O) vs CIT (FCR or BR) arms1*
*CLL-13 was a multicentre, open-label, Phase III, multiple arm study for first-line treatment of fit CLL patients. The study evaluated whether standard chemoimmunotherapy (FCR, BR) in frontline treatment of physically fit CLL patients can be replaced by combinations of targeted drugs (venetoclax) with anti-CD20-antibodies (obinutuzumab). Treatment with VEN+O was completed after twelve 28-day cycles. The CLL-13 trial included two other arms which cannot be discussed as they are unlicensed in the UK.
In the primary analysis (median follow-up of 38.8 months), both co-primary endpoints were met1
†VENCLYXTO was started on Cycle 1, Day 22 and increased to full dose for Cycle 3, Day 1. VENCLYXTO was continued until the end of Cycle 12. VENCLYXTO 400 mg daily after initial dose-titration period.
‡Obinutuzumab dosing: 100 mg Cycle 1, Day 1, followed by 900 mg on Days 1 or 2; 1000 mg on Days 8 and 15 and Day 1 of subsequent cycles.
§FCR for patients ≤65 years: fludarabine 25 mg/m2 days 1-3, cyclophosphamide 250 mg/m2 days 1-3, rituximab 375 mg/m2 day 1 cycle 1 and 500 mg/m2 day 1 cycles 2-6.
¶BR for patients >65 years: bendamustine 90 mg/m2 day 1-2 of cycles 1-6, rituximab 375 mg/m2 day 1 cycle 1 and 500 mg/m2 day 1 cycles 2-6. Each cycle was 28 days.
#MRD negativity was assessed at the end of treatment. uMRD = <1 tumour cell per 104 white cells.
**Assessed using the International Workshop on CLL (iwCLL) updated National Cancer Institute-sponsored Working Group (NCI-WG) guidelines (2008).
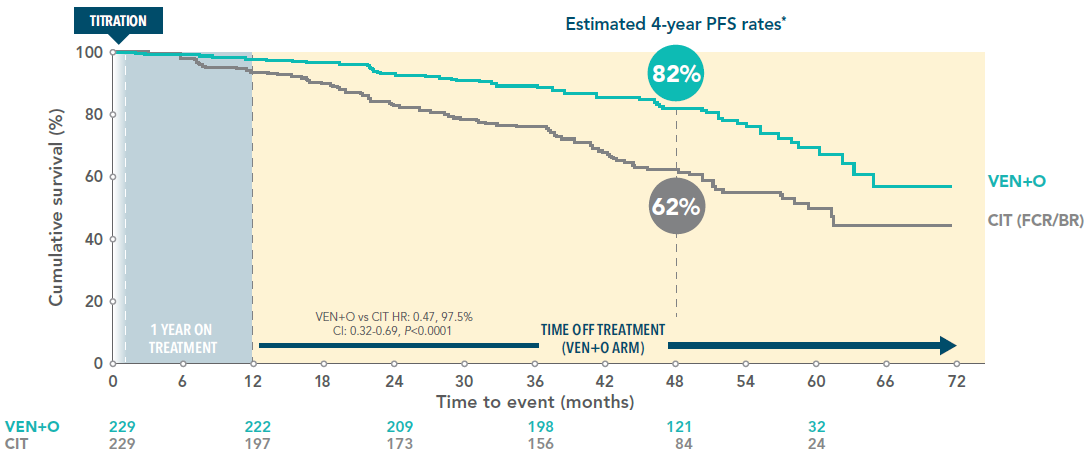
VEN+O demonstrated significantly higher PFS vs CIT (FCR/BR) 4 years after initiating VEN+O treatment
PFS of VEN+O vs CIT (ITT population; secondary endpoint)3
Median PFS:3
VEN+O: not reached
CIT: 59.4 months
Co-primary endpoint of PFS between relevant arms was met at the primary analysis after median follow-up 38.8 months. At the primary analysis, the secondary endpoint of PFS between VEN+O and CIT was also met (p<0.001).1
Adapted from Fürstenau M et al. 2024.
Data cutoff January 2023.
*Median observation time was 50.7 months.
The CLL-13 trial included other arms, which cannot be discussed as they are unlicensed in the UK.
Rate of INV-assessed CR* 3 months after completing treatment (ITT population; secondary endpoint)1†
Adapted from Eichhorst B et al. 2023.
First data cutoff February 2021.
*Complete response rate is CR+CRi (secondary endpoint).
†Median follow-up 38.8 months.
‡ORR=CR+CRi+PR (secondary endpoint).
Co-primary endpoint of uMRD between VEN+O and CIT arms was met:1
At month 15, a significantly higher percentage of patients in the VEN+O arm had uMRD compared to the CIT arm (86.5% [97.5% CI: 80.6–91.1] vs. 52.0% [97.5% CI: 44.4–59.5]; p<0.001)
For full safety information, please refer to the SmPC
AEs, Adverse events; BR, Bendamustine–rituximab; CI, Confidence Interval; CIRS, Cumulative illness rating scale; CIT, Chemoimmunotherapy; CLL, Chronic lymphocytic leukaemia; CR, Complete response; CrCl, Creatine clearance; CRi, CR with incomplete marrow recovery; CTC, Common terminology criteria; del(17p), 17p deletion; FCR, fludarabine–cyclophosphamide–rituximab; HR, Hazard ratio; INV, Investigator; ITT, Intent-to-treat; IV, Intravenous; iwCLL, International workshop on CLL; MRD, Minimal residual disease; NCI-WG, National Cancer Institute-sponsored working group; ORR, Overall response rate; PFS, Progression-free survival; PR, Partial response; TLS, Tumour lysis syndrome; TP53, Tumour protein 53; TTnT, Time to next treatment; uMRD, Undetectable minimal residual disease; VEN+O, VENCLYXTO + Obinutuzumab; 1L, First line.
References
- Eichhorst B et al. N Engl J Med. 2023; 388: 1739-54.
- VENCLYXTO Summary of Product Characteristics.
- Fursentau M et al. Lancet Oncol. 2024. 25(6): 744-59.
UK-VNCCLL-250052. Date of preparation: March 2025.
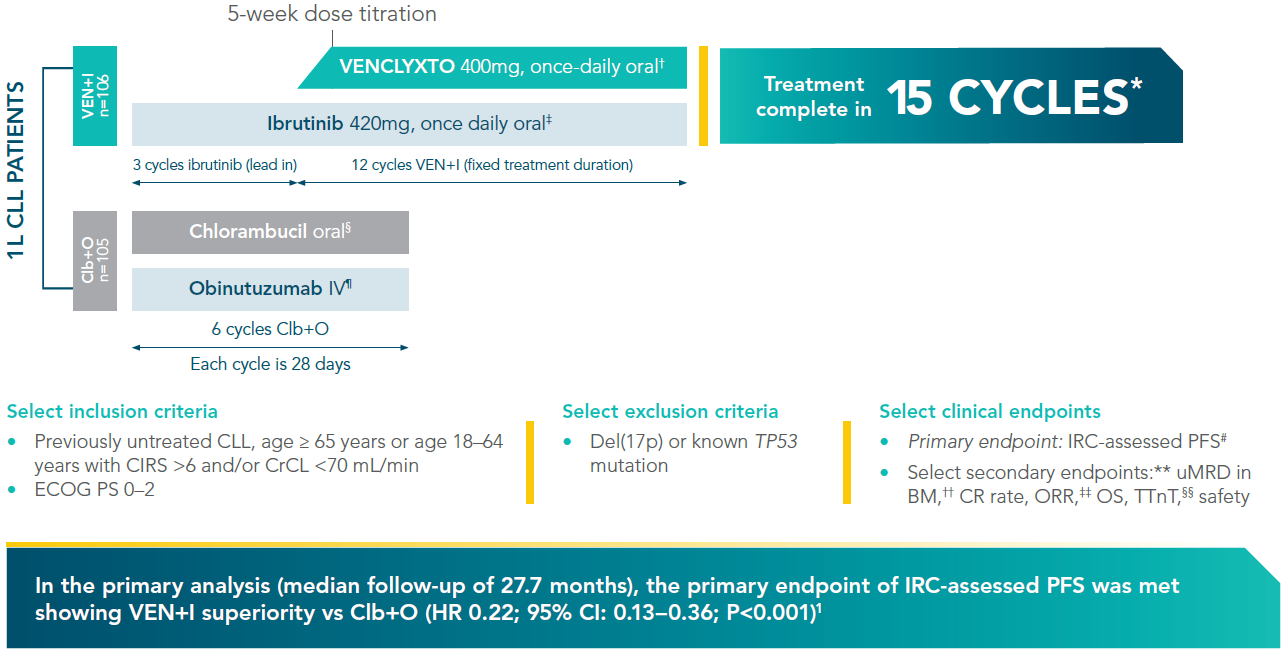
VENCLYXTO + ibrutinib in 1st line CLL
VENCLYXTO + ibrutinib (VEN+I) vs chlorambucil + obinutuzumab (Clb+O)1,2*
*GLOW was a randomised, open-label, Phase III trial evaluating the efficacy and safety of VEN+I versus Clb+O in untreated CLL patients. Treatment in the VEN+I arm was completed after fifteen 28-day cycles.
†VENCLYXTO was initiated on day 1 of cycle 4 with dose ramp-up over 5 weeks (20, 50, 100, 200, and 400 mg/day) and continued at 400 mg/day from cycle 5 onward.
‡Ibrutinib 420 mg daily was administered as a single agent for the first 3 cycles, followed by VENCLYXTO in combination with ibrutinib for twelve 28-day cycles.
§Chlorambucil dosing: 0.5 mg/kg body weight on Days 1 and 15 of cycle 1–6.
¶Obinutuzumab dosing: 1000 mg on Days 1 (or 100 mg on Day 1 and 900 mg on Day 2), 8 and 15 in cycle 1. In cycles 2–6, 1000 mg was given on Day 1.
#Defined as time from randomisation to disease progression or death from any cause, whichever occurred first.
**The statistical testing hierarchy for secondary endpoints, in order, was uMRD rate in bone marrow, CR rate, OR rate, and OS.
††Defined as the proportion of patients who are MRD-negative in the bone marrow (<1 CLL cell per 104 leukocytes; or <0.01%).
‡‡Defined as the proportion of patients who achieve a response (CR, CRi, nPR and PR).
§§Measured from the date of randomisation to start date of any antileukemic treatment subsequent to study treatment.
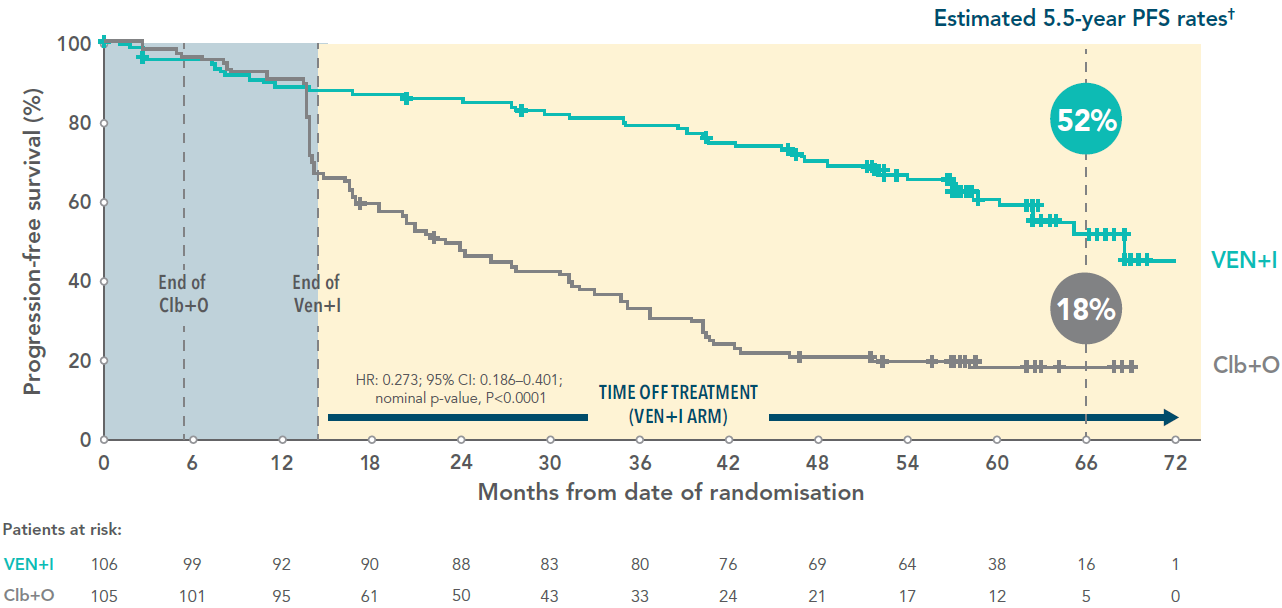
Almost three times as many VEN+I patients remained progression free vs Clb+O at 5.5-year follow-up3
PFS for VEN+I vs Clb+O at 5.5 years (primary endpoint)3*
Adapted from Niemann C et al. 2024.
*In the primary analysis at 27.7 months in the ITT population, PFS with VEN+I was superior vs Clb+O (HR 0.22; 95% CI: 0.13–0.36; P<0.001).1
†Median follow-up 67 months
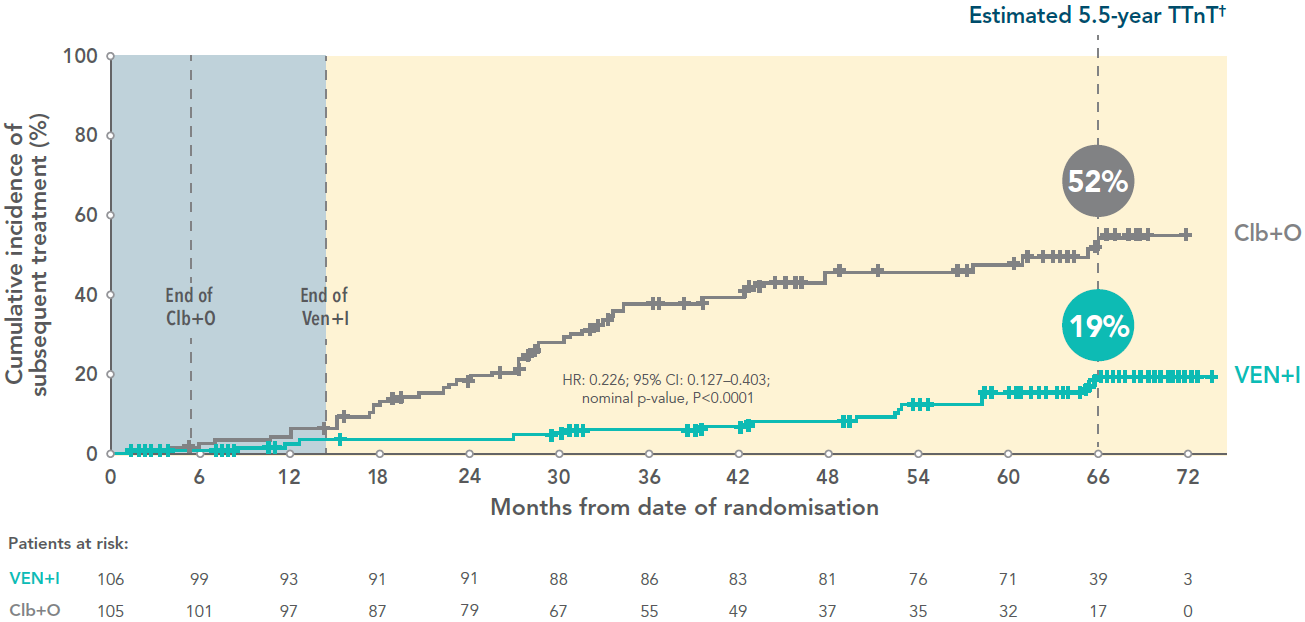
At 5.5 years, 19% of of VEN+I patients required subsequent treatment vs 52% for Clb+O patients3
Time to next treatment* for VEN+I vs Clb+O at 5.5 years (secondary endpoint)3
Adapted from Niemann C et al. 2024.
*Time to next treatment was defined as the time from randomisation to the start of any subsequent anticancer therapy; deaths were censored.
†Median follow-up 67 months
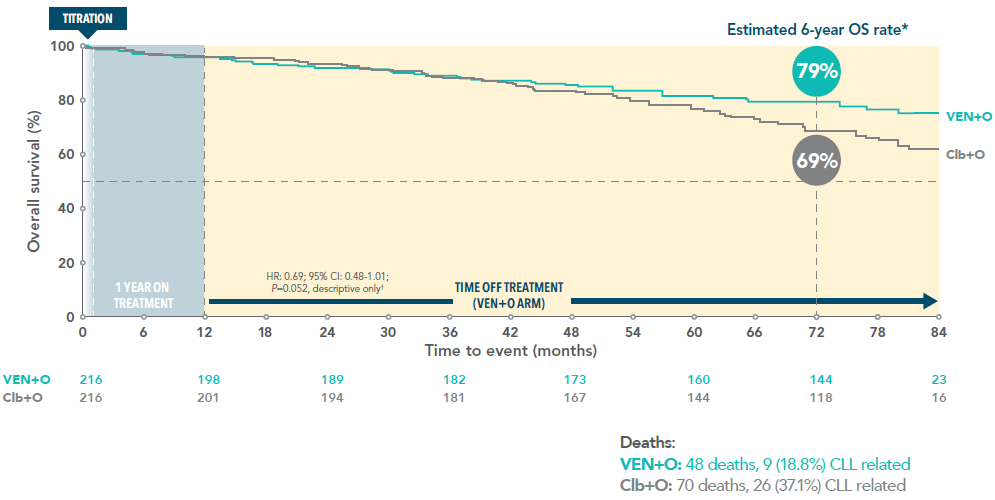
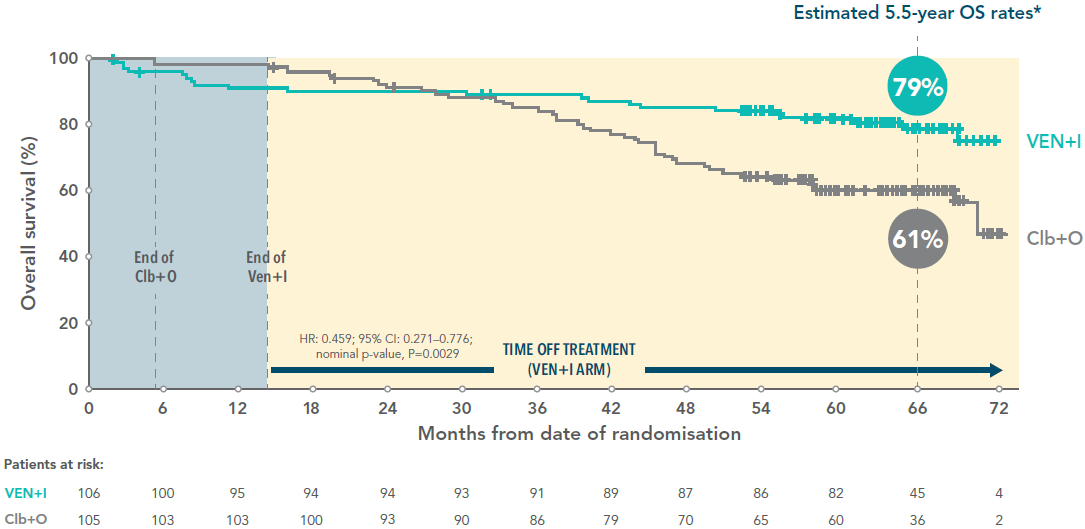
Over 75% overall survival for VEN+I patients 5.5 years after initiating treatment3
5.5-year-overall survival rate of VEN+I vs Clb+O (secondary endpoint)3
In the primary analysis at a median follow-up of 27.7 months, testing of OS was exploratory. No difference between VEN+I and Clb+O was observed (HR: 1.048 [95% CI: 0.454-2.419])1†
Adapted from Niemann C et al. 2024.
†The hypothesis for a secondary endpoint was tested only if the null hypotheses for the primary endpoint and preceding secondary endpoint(s) were rejected. The statistical testing hierarchy for secondary endpoints, in order, was uMRD rate in bone marrow, complete response rate, overall response rate, and overall survival.
*Median follow-up 67 months
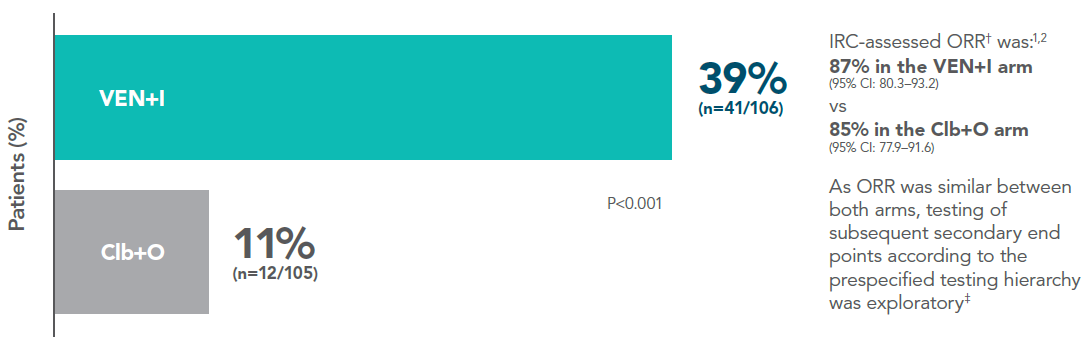
Data from primary analysis, median follow-up was 27.7 months (range 1.7–33.8 months). Response rates by IRC (ITT population; secondary endpoints)1
Complete response (CR+CRi) rate*
Adapted from Kater A et al. 2022.
*Complete response rate is CR + CRi (secondary endpoint).
†Overall Response Rate = CR+CRi+nPR+PR (secondary endpoint).
‡The hypothesis for a secondary endpoint was tested only if the null hypotheses for the primary endpoint and preceding secondary endpoints were rejected.
The statistical testing hierarchy for secondary endpoints, in order, was uMRD rate in bone marrow, CR rate, ORR, and OS.
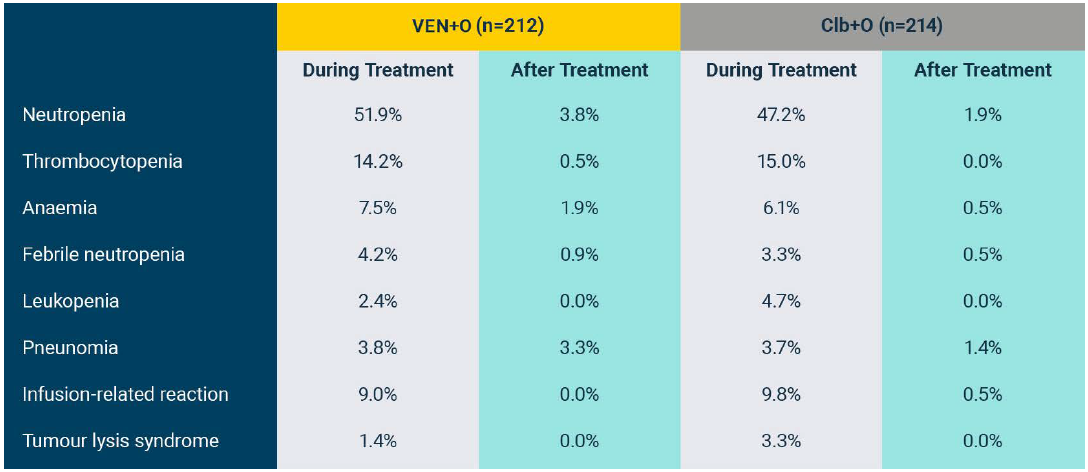
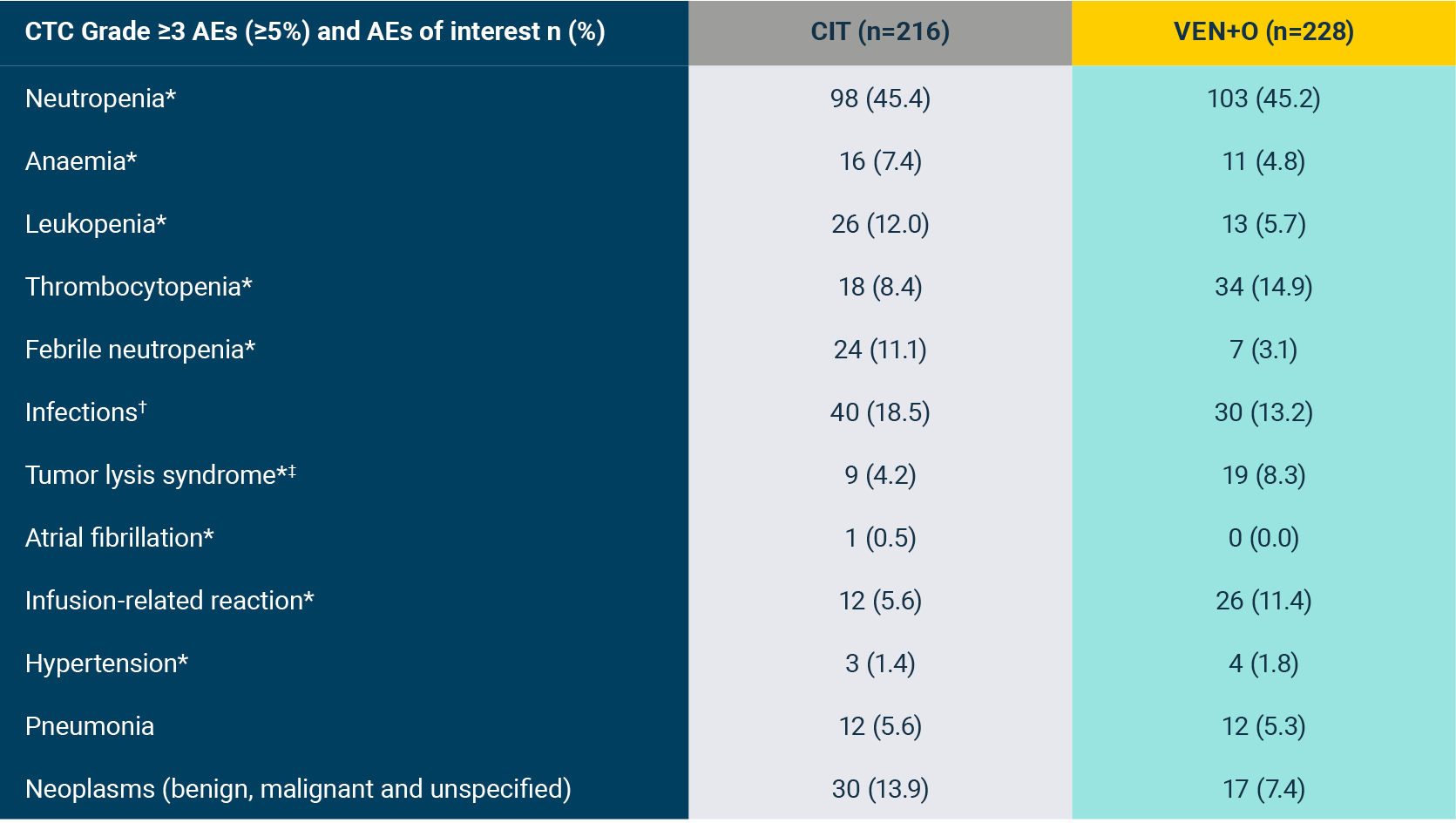
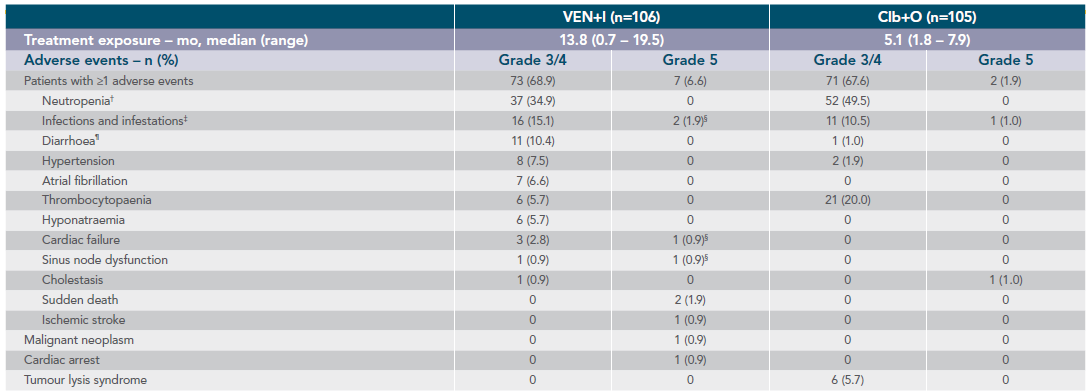
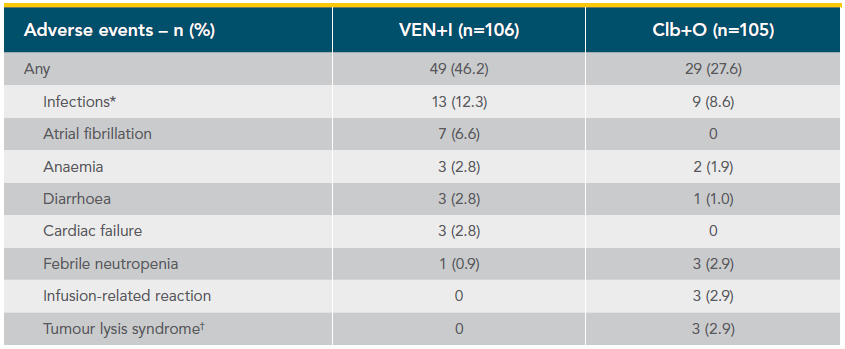
Grade 3/4 AEs occurring in ≥5% of either arm and Grade 5 AEs occurring in any patient in the GLOW study (safety population)1*
*Fifteen 28-day cycles are equivalent to 13.8 months for VEN+I and six 28-day cycles are equivalent to 5.5 months for Clb+O. Patients may have had treatment exposure times exceeding these limits due to cycle holds.
†Includes ‘neutrophil count decreased’. Rates of febrile neutropenia (grade ≥3): 1.9% for VEN+I vs 2.9% for Clb+O.
‡Includes multiple preferred terms. Only pneumonia (grade ≥3) occurred in 5% or more of patients in the VEN+I (7 [6.6%]) and Clb+O (6 [5.7%]) arms.
§Both grade 5 AEs were pneumonia (one patient experienced three grade 5 AEs: pneumonia, cardiac failure, and sinus node dysfunction).
¶In the VEN+I arm, 3 diarrhoea (grade ≥3) resolved or improved after a median of 9.0 days.
Adapted from Kater A et al. 2022.
Median follow-up of 27.7 months.
Serious adverse events in GLOW occurring in ≥2% of patients in either treatment arm (safety population)1
*Includes multiple preferred terms. For the VEN+I arm, these included: pneumonia (n=6 [5.7%]); bronchitis, bronchopulmonary aspergillosis, cellulitis, Clostridium colitis, erysipelas, gastroenteritis, gastrointestinal infection, infection, influenza, cytomegaloviral pneumonia, septic shock, tonsillitis (all n=1 [0.9%]; patients may have had more than one adverse event). For the Clb+O arm, these included: pneumonia (n=6 [5.7%]); Escherichia urinary tract infection, pneumococcal sepsis, respiratory tract infection, urinary tract infection (all n=1 [1.0%]).
†By Howard criteria.
Adapted from Kater A et al. 2022.
Adverse events are reported by Medical Dictionary for Regulatory Activities (MedDRA) superclass and preferred terms and the National Cancer Institute Common Terminology
Criteria for Adverse Events grade.
Median follow-up of 27.7 months.
For full safety information, please refer to the SmPC
AEs, Adverse events; BM, Bone marrow; CI, Confidence interval; CIRS, Cumulative illness rating scale; Clb+O, Chlorambucil + obinutuzumab; CLL, Chronic lymphocytic leukaemia; CrCl, Creatine clearance; CR, Complete response; CRi, CR with incomplete haematologic recovery; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, Hazard ratio; IRC, Independent review committee; ITT, Intent-to-treat; MRD, Minimal residual disease; nPR, Nodular partial response; ORR, Overall response rate; OS, Overall survival; PFS, Progression-free survival; PR, Partial response; SmPC, Summary of Product Characteristics; TP53, Tumour protein 53; TTnT, Time-to-next treatment; uMRD, Undetectable minimal residual disease; VEN+I, VENCLYXTO + ibrutinib.
References
- Kater A et al. N Engl J Med. 2022; 1(7): DOI: 10.1056/EVIDoa2200006 (and Suppl.).
- VENCLYXTO Summary of Product Characteristics.
- Niemann C et al. Presented at ASH 2024. Oral presentation #1871.
UK-VNCCLL-250048. Date of preparation: March 2025.
VENCLYXTO + ibrutinib in 1st line CLL
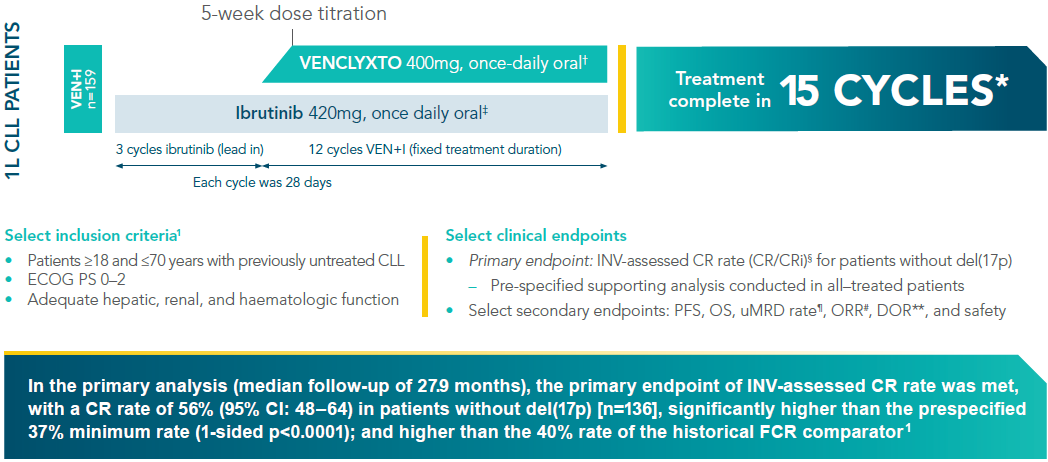
VENCLYXTO + ibrutinib (VEN+I) in fit patients with previously untreated CLL (FD Cohort)1,2*
*CAPTIVATE (FD Cohort) was an open-label, multicentre, single-arm, Phase II study evaluating fixed-duration VEN+I in 1L treatment of CLL. Treatment was completed after fifteen 28-day cycles.
†VENCLYXTO was initiated on day 1 of cycle 4 with dose ramp-up over 5 weeks (20, 50, 100, 200, and 400 mg/day) and continued at 400 mg/day from cycle 5 onward.
‡Ibrutinib 420 mg daily was administered as a single agent for the first 3 cycles, followed by VENCLYXTO in combination with ibrutinib for twelve 28-day cycles.
§Assessed using the iwCLL criteria (2008).
¶Defined as proportion of patients who are MRD negative (<1 CLL cell per 104 leukocytes).
#Defined as proportion of patients with PR or better, as per iwCLL criteria.
**Defined as time from initial documentation of response until PD or death from any cause.
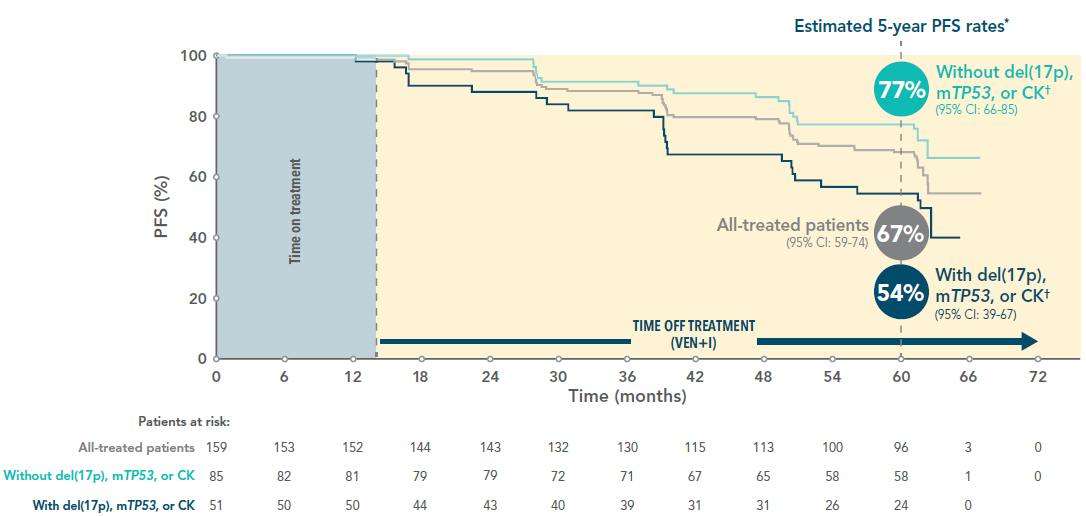
The majority of all-treated patients had not progressed and were off treatment at 5 years3*
5-year PFS rate in all-treated patients and by del(17p), mTP53 or CK† status
Adapted from Wierda W et al. 2024.
*Median time on study: 61.2 months (range, 0.8–66.3).
†CK defined as ≥3 chromosomal abnormalities by conventional CpG-stimulated cytogenetics.
Landmark estimate of TTnT at 4 years was 84% (95% CI: 77–89)4*
- At 4-year follow-up, median TTnT was not reached (n=28; range: 1–53 months)4
*Median time on study 49.8 months (range, 0.8–53.1).
>90% overall survival for all-treated patients at 5-year follow-up3*
5-year OS rate in all-treated patients and by del(17p), mTP53 or CK† status
Adapted from Wierda W et al. 2024.
*Median time on study: 61.2 months (range, 0.8–66.3).
†CK defined as ≥3 chromosomal abnormalities by conventional CpG-stimulated cytogenetics.
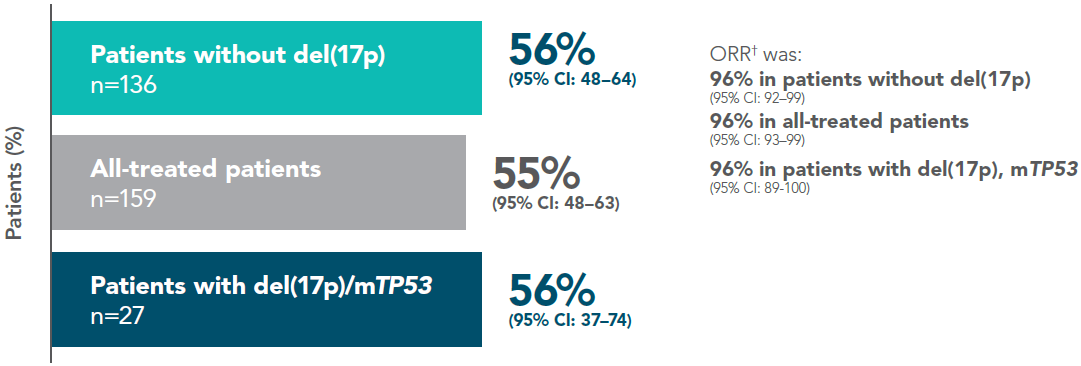
INV-assessed CR rate* (CR/CRi) in patients without del(17p), (FD Cohort; primary endpoint)1
In the primary analysis (median follow-up of 27.9 months), the primary endpoint of INV-assessed CR rate was met, with a CR rate of 56% (95% CI: 48–64) in patients without del(17p) [n=136], significantly higher than the prespecified 37% minimum rate (1-sided p<0.0001); and higher than the 40% rate of the historical FCR comparator.1
Adapted from Tam C et al. 2022.
Median observation time of 27.9 months.
*Proportion of patients with CR or CRi, assessed using iwCLL criteria (2008).
†Defined as proportion of patients with PR or better, as per iwCLL criteria.
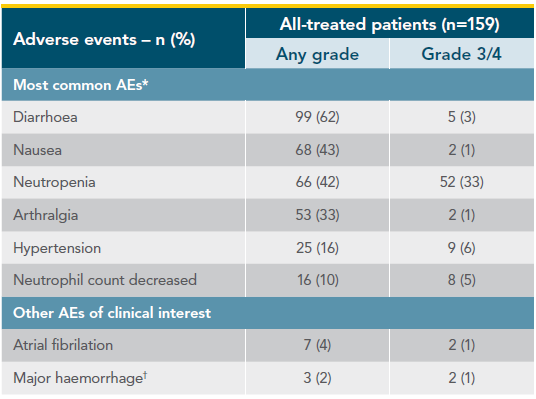
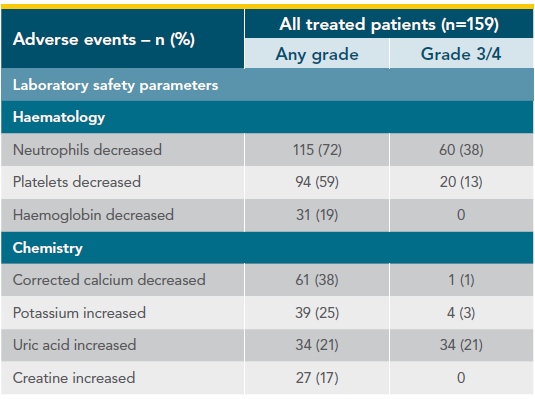
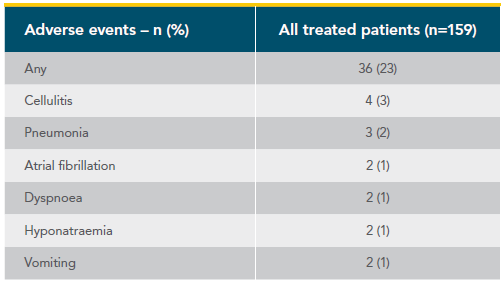
Treatment-emergent adverse events in CAPTIVATE (FD Cohort)1,2
For full safety information, please refer to the SmPC
AEs, Adverse events; CI, Confidence interval; CLL, Chronic lymphocytic leukaemia; CK, Complex karyotype; CR, Complete response; CRi, CR with incomplete haematologic recovery; DOR, Duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; FCR, Fludarabine, cyclophosphamide, rituximab; FD, Fixed-duration; INV, Investigator; iwCLL, International Workshop on CLL; MedDRA, Medical Dictionary for Regulatory Activities; MRD, Minimal residual disease; mTP53, mutated TP53; ORR, Overall response rate; OS, Overall survival; PD, Progressive disease; PFS, Progression-free survival; PR, Partial response; SmPC, Summary of Product Characteristics; TP53, Tumour protein 53; TTnT, Time-to-next treatment; uMRD, Undetectable minimal residual disease; VEN+I, VENCLYXTO + ibrutinib; 1L, First-line.
References
- Tam C et al. Blood. 2022; 139(22): 3278–89 (and Suppl.).
- VENCLYXTO Summary of Product Characteristics.
- Wierda W et al. Presented at ASCO 2024; Oral Presentation and Poster #7009.
- Tedeschi A et al. Presented at EHA 2023; Oral Presentation and Poster #617.
UK-VNCCLL-250049. Date of preparation: March 2025.
You are advised to read the Prescribing Information and Summary of Product Characteristics to evaluate patient suitability for VENCLYXTO.
VENCLYXTO PRESCRIBING INFORMATION
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.