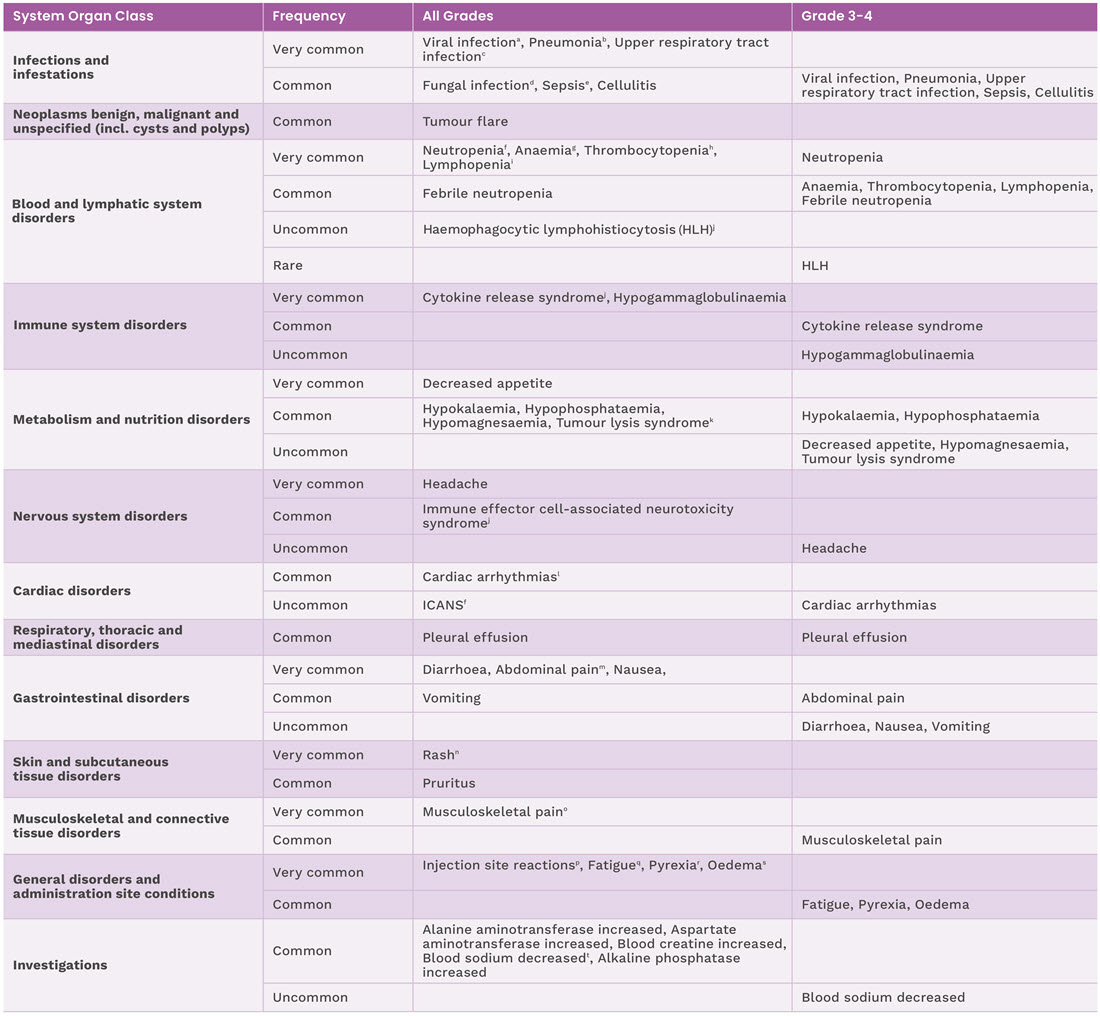
Adverse reactions reported in patients in EPCORETM NHL-11
The safety of Tepkinly was evaluated in a non-randomised, single-arm study in 382 patients with R/R LBCL (n=167), follicular lymphoma (n=129) and follicular lymphoma (3-step step-up dose schedule n=86) after 2 or more lines of systematic therapy and included all the patients who enrolled to the 48 mg dose and received at least one dose of Tepkinly. The following adverse reactions have been reported with Tepkinly during clinical studies and post marketing experience.1
For full safety information, please refer to the SmPC
Tepkinly has a generally manageable safety profile1
- 6.8% of patients in EPCORETM NHL-1 trial discontinued subcutaneous Tepkinly due to adverse reactions. Discontinuation of epcoritamab due to pneumonia occurred in 14 (3.7%) patients, viral infection in 8 (2.1%) patients, fatigue in 2 (0.5%) patients and CRS, ICANS, or diarrhoea occurred in 1 (0.3%) patient each.
- Serious adverse reactions occurred in 50% of patients.
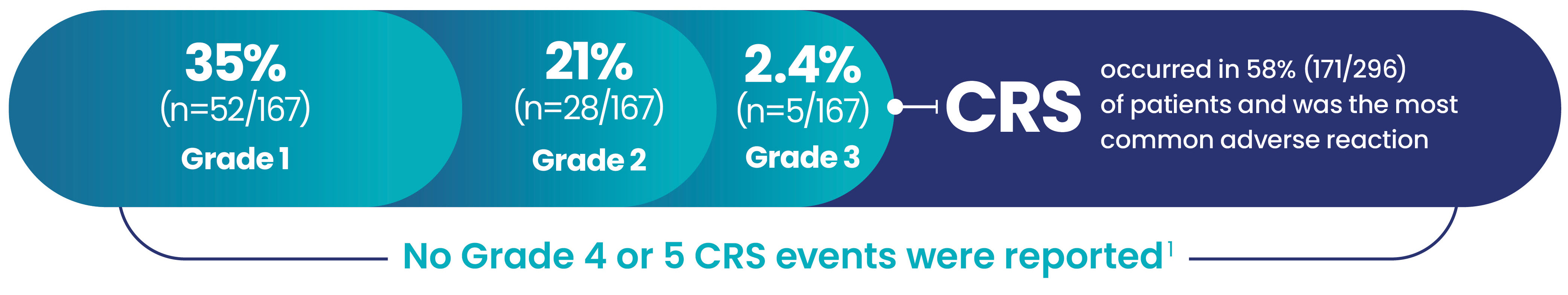
- The most common serious adverse reaction (≥10%) was CRS (34%). Fourteen patients (3.7%) experienced a fatal adverse reaction (pneumonia in 9 (2.4%) patients, viral infection in 4 (1.0%) patients, and ICANS in 1 (0.3%) patient).
Adverse reactions for Tepkinly from clinical studies are listed by MedDRA system organ class and are based on the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); and very rare (<1/10,000).
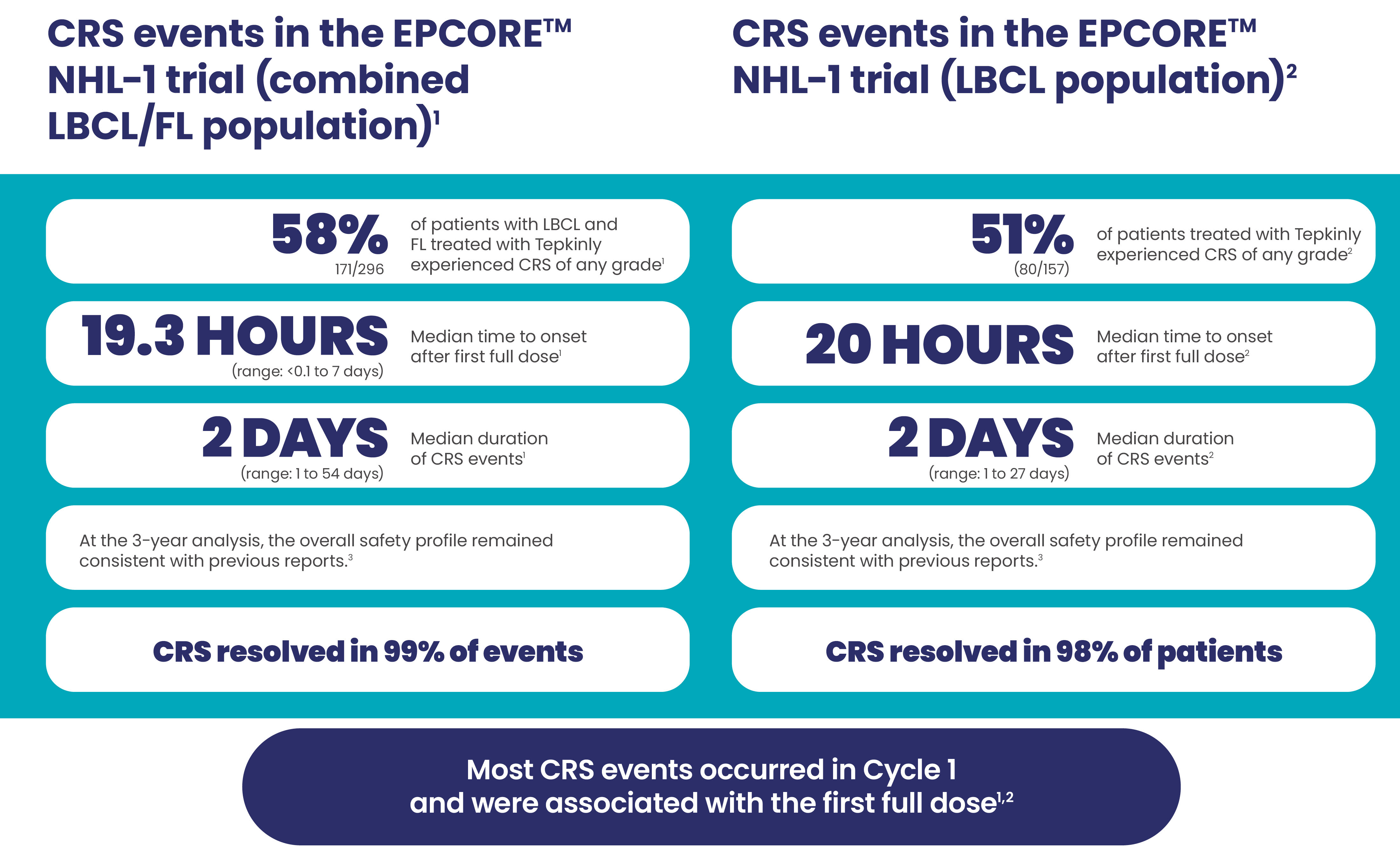
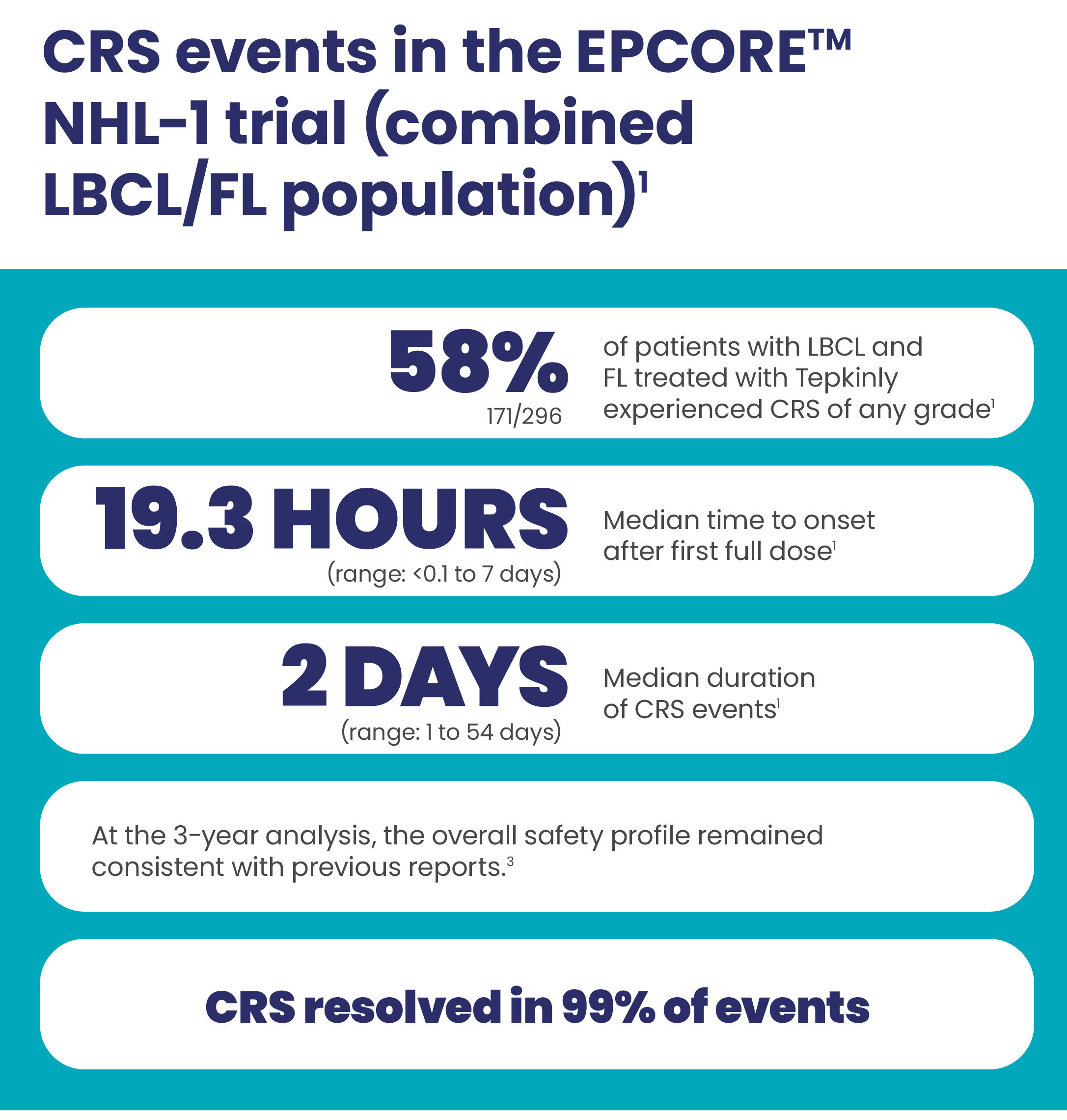
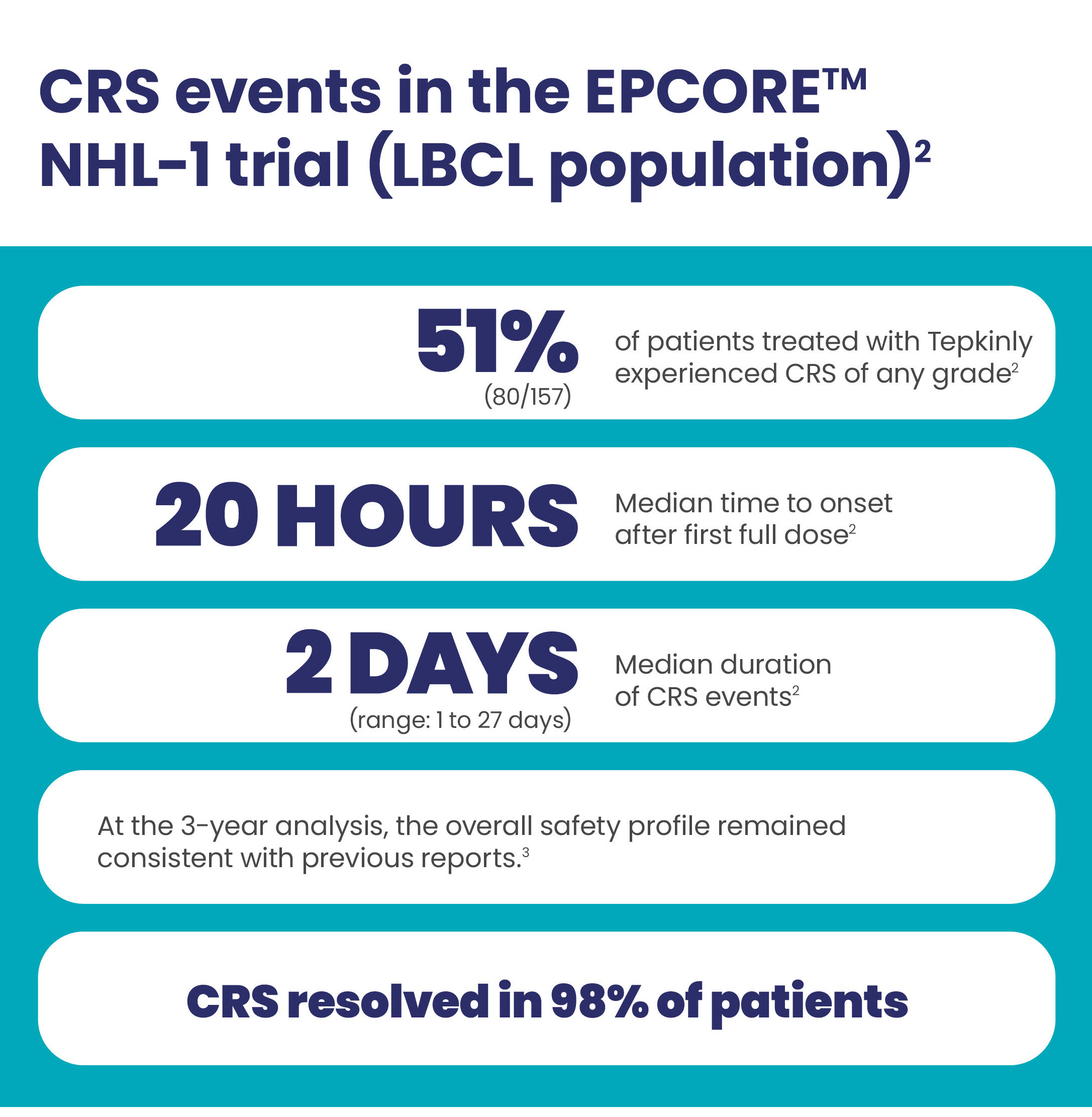
CRS events with Tepkinly1-4
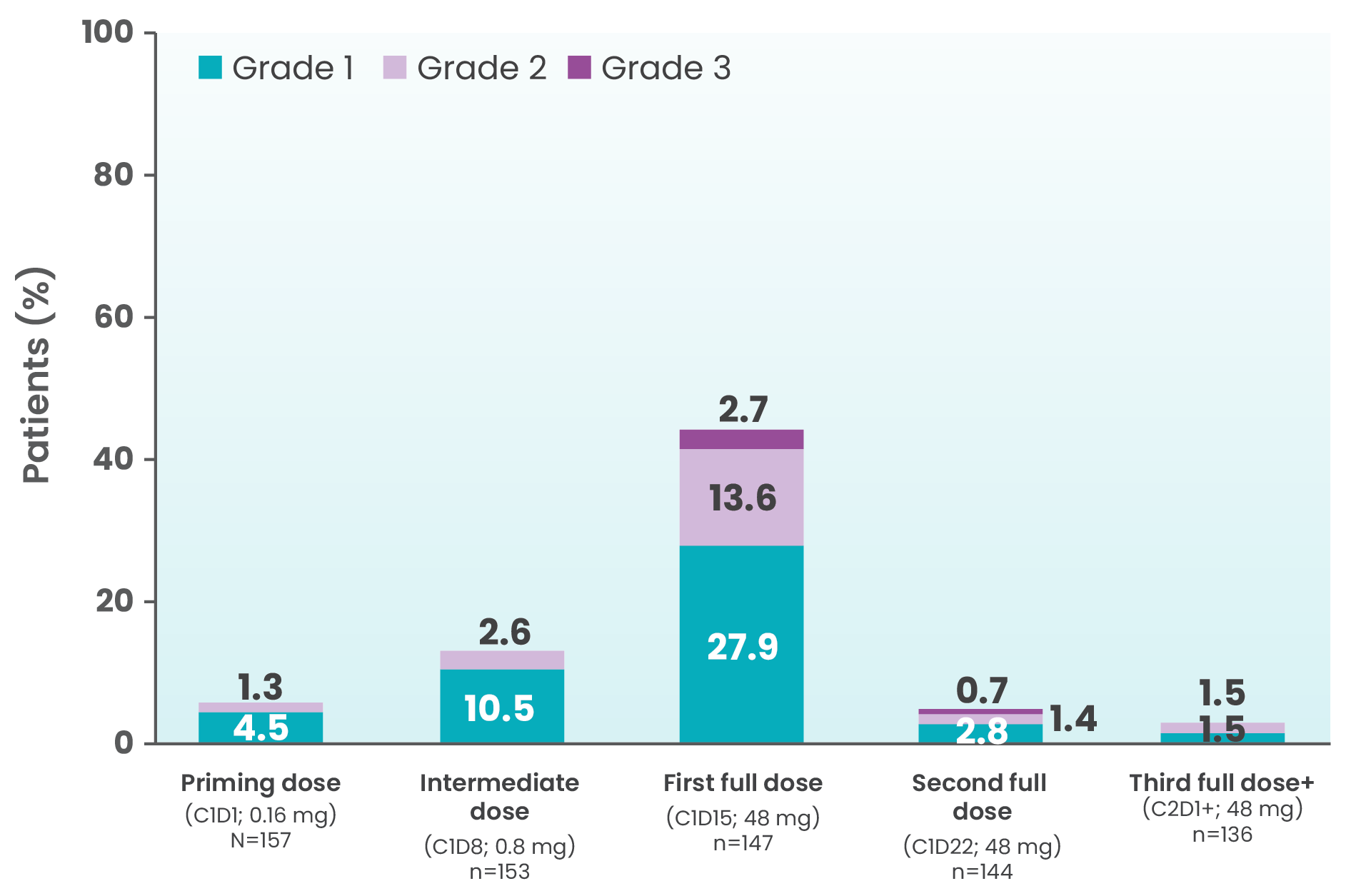
CRS events by dosing period at 2-year follow-up (full cohort, R/R LBCL, N=157)4
Patients should be monitored for signs and symptoms of CRS following Tepkinly administration, and managed per current practice guidelines.
Patients should be hospitalised for 24 hours after administration of the Cycle 1 Day 15 dose of 48 mg to monitor for signs and symptoms of CRS.
At the first signs or symptoms of CRS, immediately evaluate patient and provide supportive therapy based on severity. Advise patients to contact their healthcare provider if they develop any neurological signs or symptoms, and that the onset of events may be delayed. Delay or discontinue Tepkinly as recommended.
CRS was generally low grade1
Haemophagocytic lymphohistiocytosis (HLH)1
Haemophagocytic lymphohistiocytosis (HLH), including fatal cases, have been reported in patients receiving epcoritamab. HLH is a life-threatening syndrome characterised by fever, skin rash, lymphadenopathy, hepato- and/or splenomegaly and cytopenias. HLH should be considered when the presentation of CRS is atypical or prolonged. Patients should be monitored for clinical signs and symptoms of HLH. For suspected HLH, epcoritamab must be interrupted for diagnostic workup and treatment for HLH initiated. If HLH is confirmed, administration of Tepkinly should be discontinued.
ICANS occurrence with Tepkinly1
 ICANS, including fatal events, have occurred in patients receiving Tepkinly1
ICANS, including fatal events, have occurred in patients receiving Tepkinly1
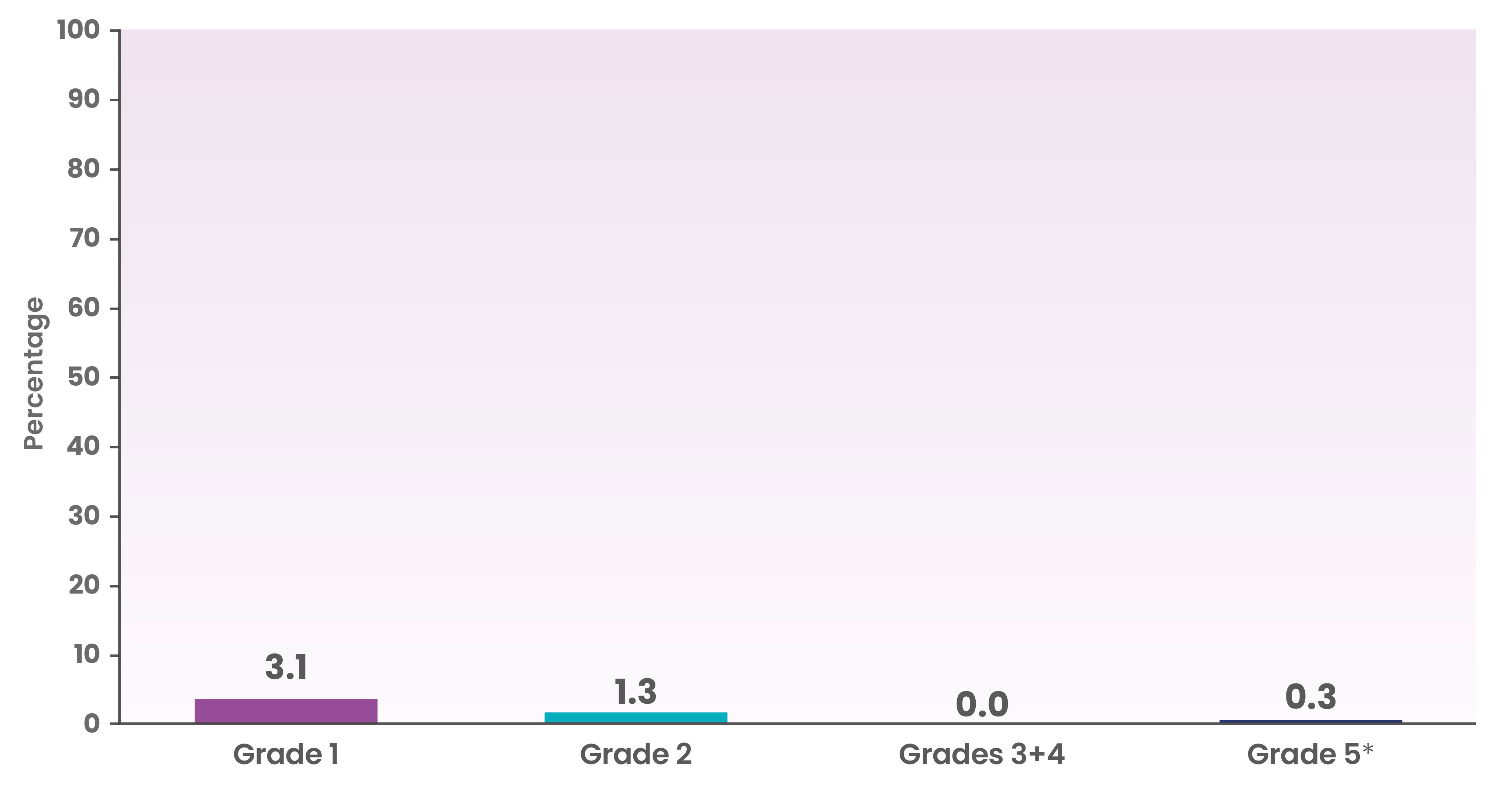
ICANs occurred in 4.7% (18/382) of patients in the NHL-1 trial1
Occurrence of ICANS by grade in the NHL-1 trial
- ICANS including fatal events have occurred in patients receiving Tepkinly
- The median time to first ICANS onset from the start of Tepkinly treatment was 18 days (range: 8-141 days)
- Dose delays due to ICANS occurred in 1.0% of patients
- ICANS resolved in 94% (17/18) of patients with supportive care
- The median time to resolution of ICANS was 2 days (range: 1-9 days)
At the 3 year update, the incidence and severity of ICANS remained unchanged since previous reports3
Patients should be monitored for signs and symptoms of ICANS following Tepkinly administration. Patients should be hospitalised for 24 hours after administration of the Cycle 1 Day 15 dose of 48 mg to monitor for signs and symptoms of ICANS1
Epcoritamab has major influence on the ability to drive and use machines. Due to the potential for ICANS, patients receiving epcoritamab are at risk of altered level of consciousness. Patients should be advised to exercise caution (or avoid if symptomatic) while driving, cycling or using heavy or potentially dangerous machines.1
Management of ICANs while using Tepkinly1
Epcoritamab has major influence on the ability to drive and use machines. Due to the potential for ICANS, patients receiving epcoritamab are at risk of altered level of consciousness. Patients should be advised to exercise caution (or avoid if symptomatic) while driving, cycling or using heavy or potentially dangerous machines.1
Serious infections of any grade occurred in 25% (n=42/167) of patients treated with Tepkinly1
- Serious or fatal infections were observed in patients treated with epcoritamab in clinical studies
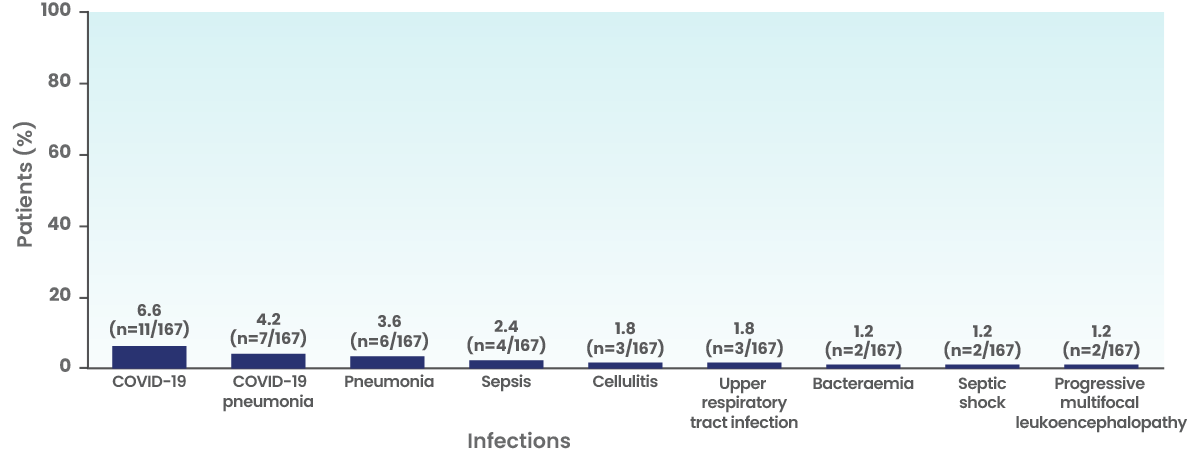
- The most frequent serious infections were COVID-19, COVID-19 pneumonia, pneumonia, sepsis, cellulitis, upper respiratory tract infection, bacteraemia, septic shock and progressive multifocal leukoencephalopathy†
Frequency of serious infections
Fatal serious infections occurred in 7 patients (4.2%)
- Median time to onset of first serious infection was 56 days (range: 4-631 days)
- Median duration of serious infection was 15 days (range 4-125 days)
- Dose delays due to serious infections occurred in 15% (n=25/167) of patients
- Treatment discontinuations due to serious infections occurred in 6% (n=10/167) of patients
- Fatal infections (Grade 5) occurred in 4.2% (n=7/167) of patients
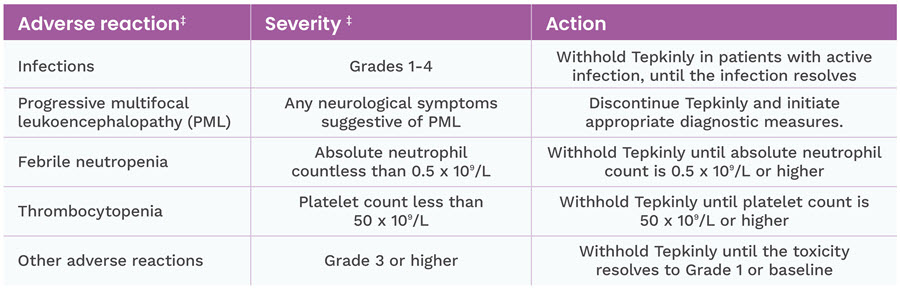
Tepkinly must not be administered in patients with active infections
Cases of progressive multifocal leukoencephalopathy (PML), including fatal cases, have been reported in patients treated with epcoritamab who have also received prior treatment with other immunosuppressive medications. If neurological symptoms suggestive of PML occur during epcoritamab therapy, treatment with epcoritamab should be discontinued and appropriate diagnostic measures initiated.1
- Exercise caution when considering the use of Tepkinly in patients with a history of recurring or chronic infections, with underlying conditions that may predispose to infections or who have had significant prior immunosuppressive treatment.
- Patients should be monitored for signs and symptoms of infection before and after Tepkinly administration, and treated appropriately.
- In the event of febrile neutropenia, patients should be evaluated for infection and managed with antibiotics, fluids and other supportive care, according to local guidelines.
- Tepkinly must not be administered in patients with active infections.
- Cases of progressive multifocal leukoencephalopathy (PML), including fatal cases, have been reported in patients treated with epcoritamab who have also received prior treatment with other immunosuppressive medications. If neurological symptoms suggestive of PML occur during epcoritamab therapy, treatment with epcoritamab should be discontinued and appropriate diagnostic measures initiated.
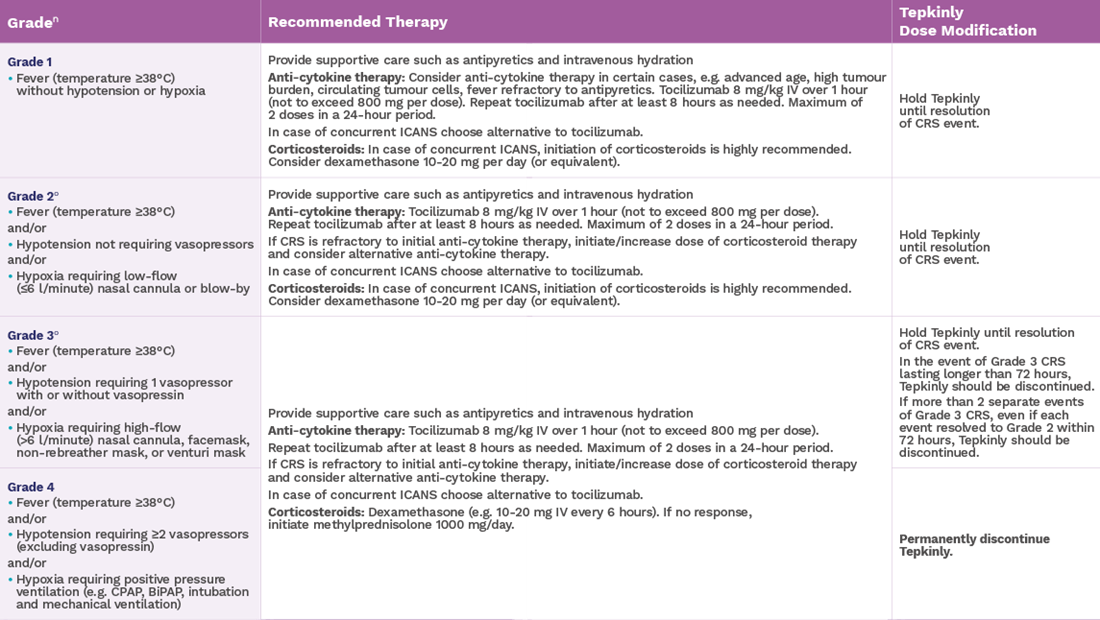
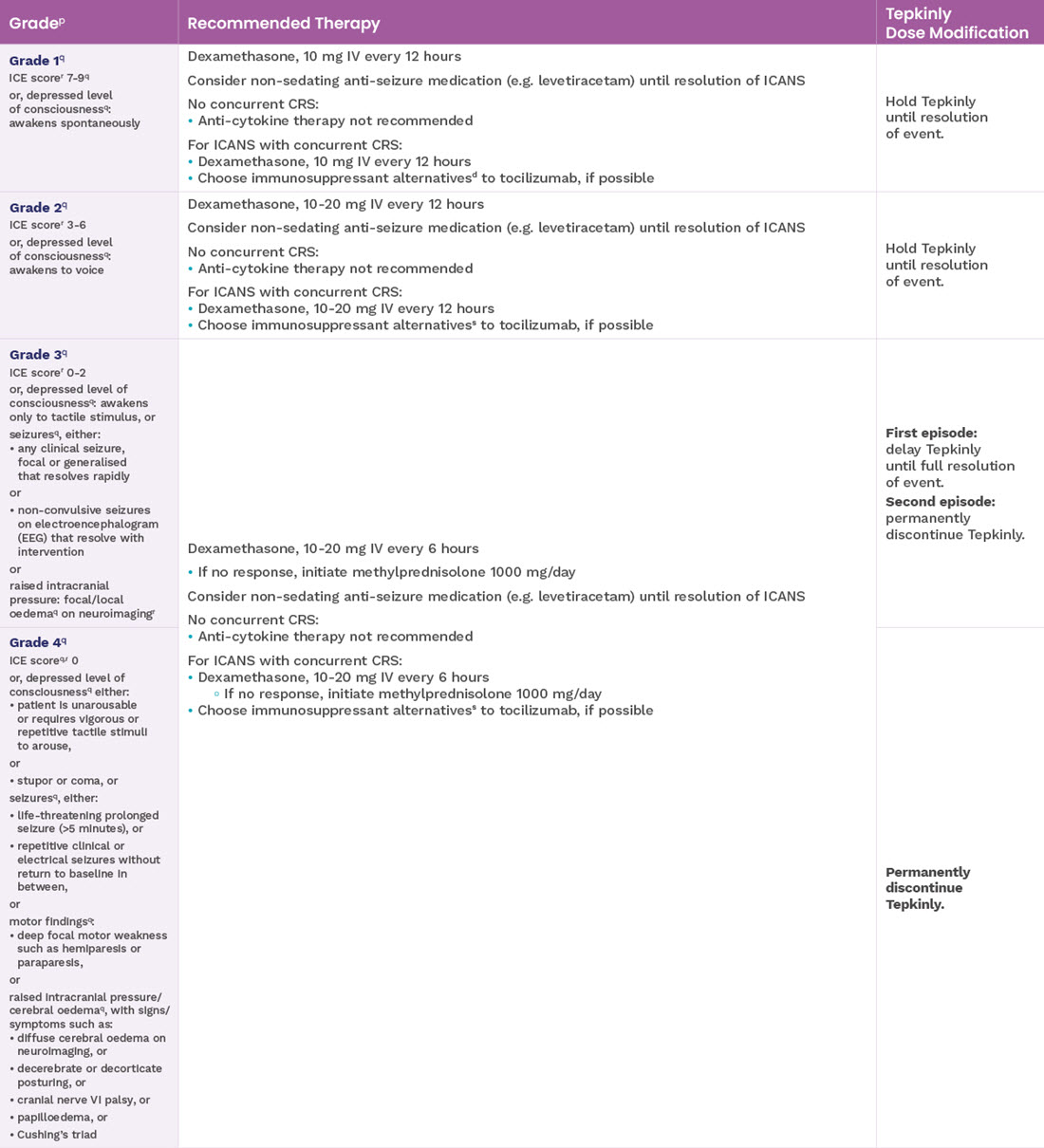
Recommended dosage modifications for adverse reactions other than ICANS and CRS1
‡Based on National Cancer Institute Common Terminology Criteria for Adverse Events.
Tumour lysis syndrome (TLS)1
TLS occurred in 1% (4/382) of patients. Median time to onset was 18 days (range 8 to 33 days), and median duration was 3 days (range 2 to 4 days).
Patients at an increased risk for TLS are recommended to receive hydration and prophylactic treatment with a uric acid lowering agent. Patients should be monitored for signs or symptoms of TLS, especially patients with high tumour burden or rapidly proliferative tumours, and patients with reduced renal function. Patients should be monitored for blood chemistries and abnormalities should be managed promptly.
Tumour Flare1
Tumour flare occurred in 1.6% (6/382) of patients, all of which were Grade 2. The median time to onset was 19.5 days (range 9 to 34 days), and median duration was 9 days (range 1 to 50 days).
Manifestations could include localised pain and swelling. Consistent with the mechanism of action of Tepkinly, tumour flare is likely due to the influx of T-cells into tumour sites following epcoritamab administration.
There are no specific risk factors for tumour flare that have been identified; however, there is a heightened risk of compromise and morbidity due to mass effect secondary to tumour flare in patients with bulky tumours located in close proximity to airways and/or a vital organ. Patients treated with Tepkinly should be monitored and evaluated for tumour flare at critical anatomical sites.
*One patient (0.6%) experienced a fatal adverse reaction (ICANS).2
†Actual EPCORE™ NHL-1 study start date: June 26, 2018. Estimated Primary Completion Date: January 2025.5
aViral infection includes COVID-19, cytomegalovirus chorioretinitis, cytomegalovirus colitis, cytomegalovirus infection, cytomegalovirus infection reactivation, gastroenteritis viral, herpes simplex, herpes simplex reactivation, herpes virus infection, herpes zoster, oral herpes, post-acute COVID-19 syndrome, and varicella zoster virus infection.
bPneumonia includes COVID-19 pneumonia and pneumonia.
cUpper respiratory tract infection includes laryngitis, pharyngitis, respiratory syncytial virus infection, rhinitis, rhinovirus infection, and upper respiratory tract infection.
dFungal infection includes candida infection, oesophageal candidiasis, oral candidiasis and oropharyngeal candidiasis.
eSepsis includes bacteraemia, sepsis, and septic shock.
fNeutropenia includes neutropenia and neutrophil count decreased.
gAnaemia includes anaemia and serum ferritin decreased.
hThrombocytopenia includes platelet count decreased and thrombocytopenia.
iLymphopenia includes lymphocyte count decreased and lymphopenia.
jEvents graded using American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria.
kClinical Tumour Lysis Syndrome was graded based on Cairo-Bishop.
lCardiac arrhythmias include bradycardia, sinus bradycardia, sinus tachycardia, supraventricular tachycardia, and tachycardia.
mAbdominal pain includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, and abdominal tenderness.
nRash includes rash, rash erythematous, rash macular, rash maculo-papular, rash popular, rash pruritic, rash pustular and rash vesicular.
oMusculoskeletal pain includes back pain, bone pain, flank pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, non-cardiac chest pain, pain, pain in extremity, and spinal pain.
pinjection site reactions include injection site bruising, injection site erythema, injection site hypertrophy, injection site inflammation, injection site mass, injection site nodule, injection site oedema, injection site pain, injection site pruritus, injection site rash, injection site reaction, injection site swelling, and injection site urticaria.
qFatigue includes asthenia, fatigue, and lethargy.
rPyrexia includes body temperature increased and pyrexia.
sOedema includes face oedema, generalised oedema, oedema, oedema peripheral, peripheral swelling, swelling, decreased and hyponatraemia. and swelling face.
tBlood sodium decreased includes blood sodium decreased and hyponatraemia.
Abbreviations
ASTCT=American Society for Transplantation and Cellular Therapy; BiPAP=bilevel positive airway pressure; C1D1=cycle 1, day 1; C1D8=cycle 1, day 8; C1D15=cycle 1, day 15; C1D22=cycle 1, day 22; C2D1+=cycle 2, days 1+; CPAP=continuous positive airway pressure; CRS=cytokine release syndrome; DLBCL=diffuse large B-cell lymphoma; ICANS=immune effector cell-associated neurotoxicity syndrome; ICE=immune effector cellassociated encephalopathy; ICP=intracranial pressure; IV=intravenous; MedDRA=Medical Dictionary for Regulatory Activities; NHL=non-Hodgkin lymphoma; SI=serious infection; TLS=tumour lysis syndrome.
References
- Tepkinly Summary of Product Characteristics.
- Vose J, et al. ASH 2023 P1729.
- Vose J, et al. ASH 2024 P4480.
- Thieblemont C et al. J Clin Oncol. 2022; 41(12): 2238-47.
By clicking the link above, you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
UK-EPCOR-260022. Date of preparation: January 2026.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com