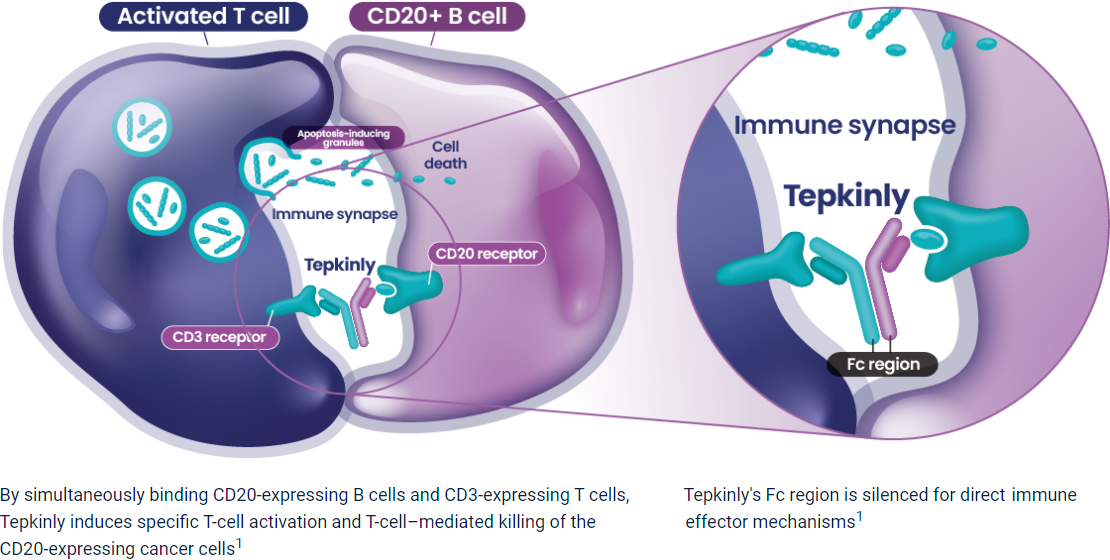
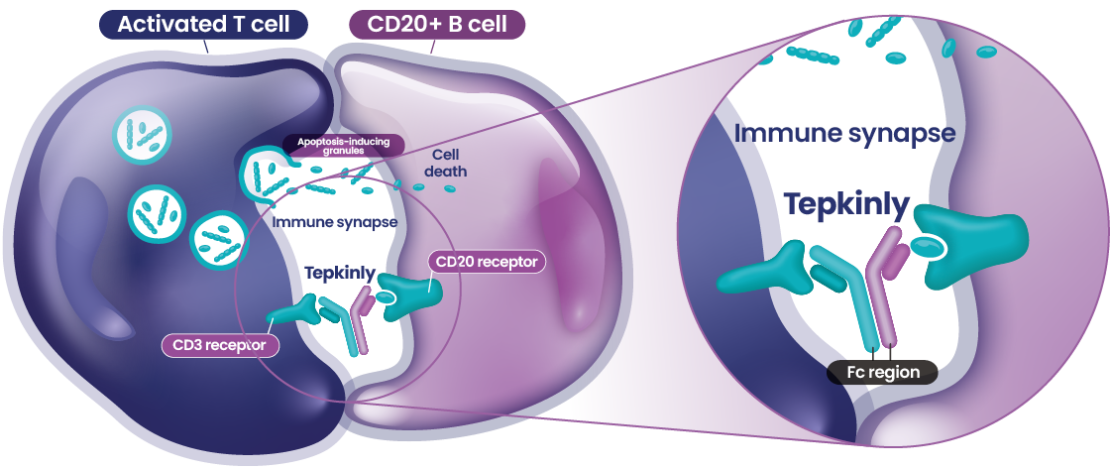
Abbreviations
3L+=third line plus; BsAb=bispecific antibody; CD3=cluster of differentiation 3; CD20=cluster of differentiation 20; DLBCL=diffuse large B-cell lymphoma; Fc region=fragment crystallization region; IgG-1=Immunoglobulin G1; NICE=National Institute for Health and Care Excellence; SMC=Scottish Medicines Consortium.
By clicking the link above, you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
UK-EPCOR-250062. Date of preparation: March 2025.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com