יעילות מוכחת בטיפול אקוטי בהתקפי מיגרנה!
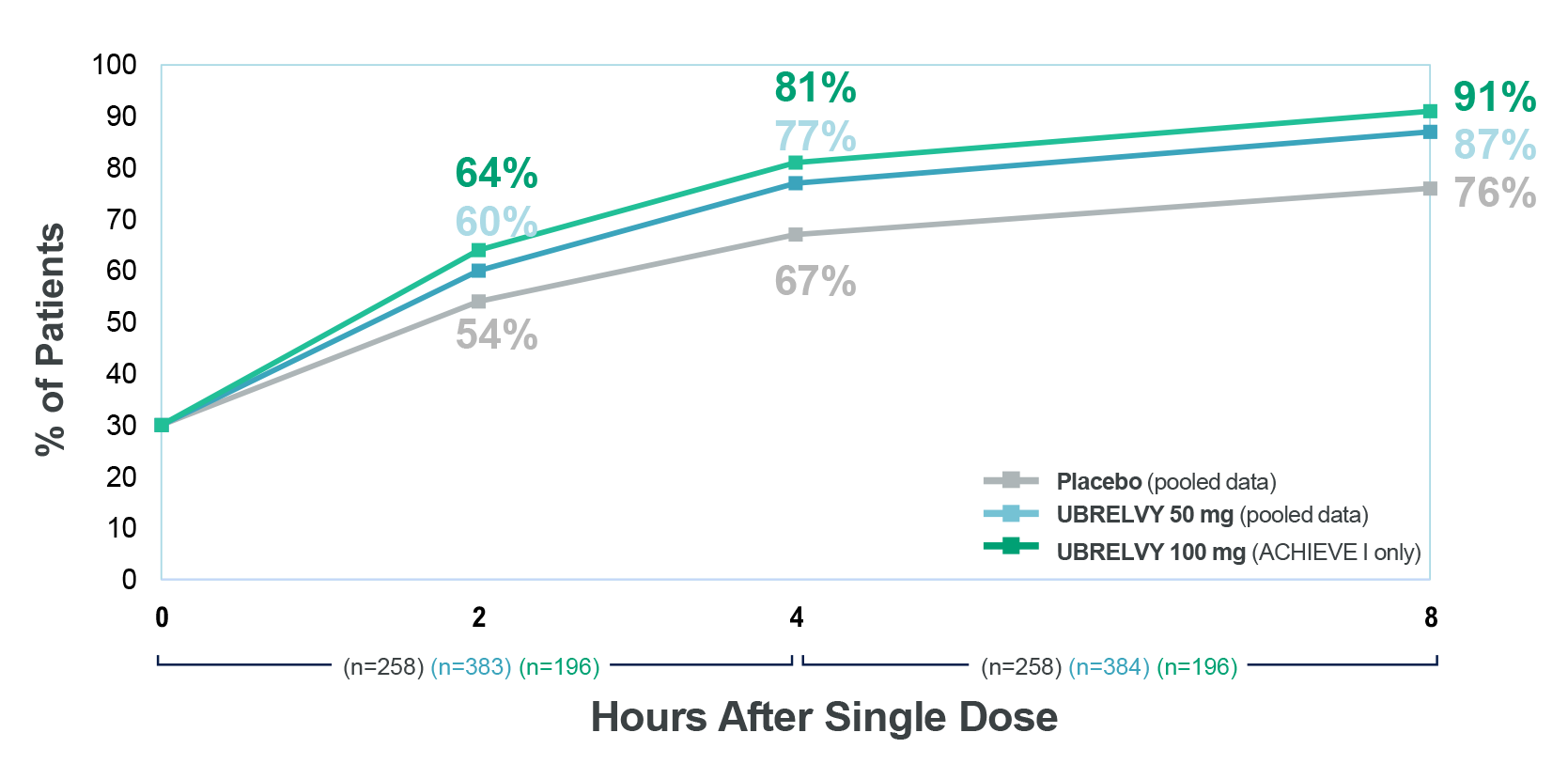
מרבית המטופלים באוברלוי חזרו לתפקוד תקין תוך שעתיים מנטילת הטיפול3
מדד תוצא נוסף: אחוז המטופלים שהשיגו חזרה לתפקוד תקין כעבור 2, 4 ו-8 שעות, עם מנה בודדת3†
Limitation: The analyses of additional endpoints were not tested in hierarchical order or adjusted for multiplicity.
Therefore, results cannot be regarded as statistically significant.
*Patients were asked to rate the performance of daily activities using 4 response options ranging from 0 (no disability, able to function normally) to 3 (severely impaired, cannot do all or most things; bed rest may be necessary). Data collected after rescue or second dose were excluded.3 †Percentages of patients achieving normal function at 2, 4, and 8 hours with a single dose were additional endpoints of the clinical trials.3
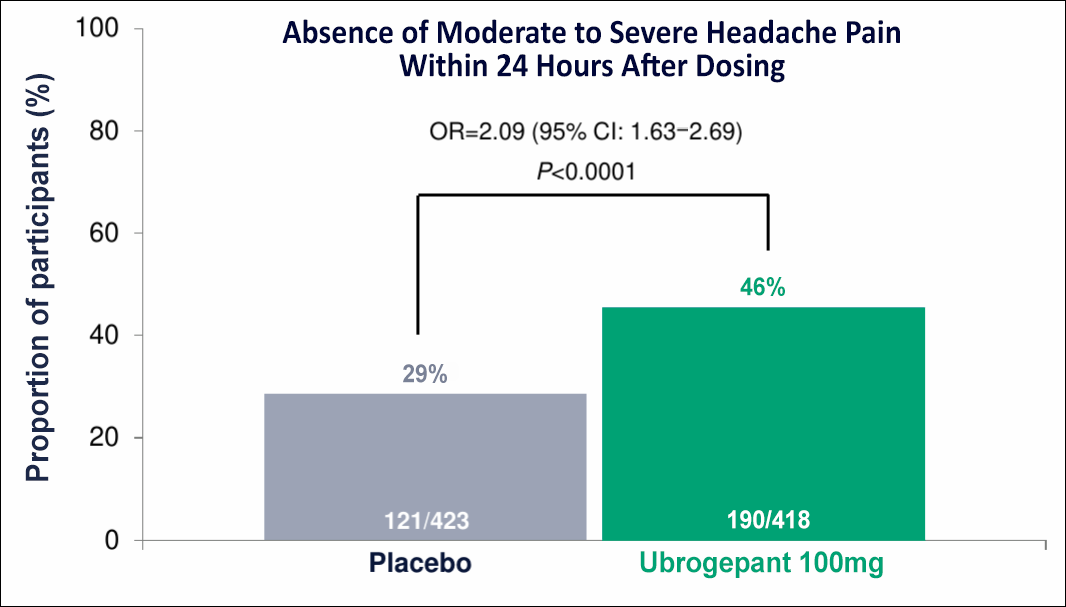
עם אוברלוי, מטופלים הצליחו לעצור את כאב הראש לפני שהתחיל!5
הטיפול עם אוברלוי במינון 100 מ"ג במהלך שלב הפרודרום במיגרנה לווה בהיעדר כאב ראש בינוני עד חמור במהלך 24 שעות לאחר מתן המנה (מדד תוצא ראשי)5
Limitation: This study evaluated a subset of the migraine population who could reliably predict the onset of a migraine attack (per clinician judgment) within a short time window. Patients should be carefully assessed on their ability to accurately predict the onset of a migraine attack and progression to the headache phase. The 50 mg dose was not assessed. Patients were not allowed to administer a second dose.
Modified intent-to-treat (mITT)=477 participants. The mITT population consists of all randomized participants with at least 1 assessment of headache occurrence within 24 hours after taking double-blind study intervention for at least 1 qualifying prodrome event during the double-blind treatment period.5
מידע נוסף על אוברלוי1
• אוברלוי נלקח דרך הפה, עם או בלי מזון, וזמין במינונים 50 ו-100 מ"ג/טבליה.
• ניתן לקחת מנה שנייה לפחות שעתיים לאחר המנה הראשונית, אם יש צורך. המינון המרבי בפרק זמן של 24 שעות הוא 200 מ"ג.
• יש לאחסן בטמפרטורה מתחת ל-25°C.
• בטיחות נטילת אוברלוי נבדקה בטיפול בעד 8 מיגרנות בתקופה של 30 יום.
• אוברלוי אינו מותווה לטיפול מניעתי נגד מיגרנה.
References:
a. Pain freedom at 2 hours (co-primary endpoint) demonstrated in 21% (50 mg: 182/886 and 100 mg: 95/448) of patients vs 13% (placebo: 119/912).
b. 60%-87% (UBRELVY 50mg) & 64%-91% (UBRELVY 100 mg) achieved normal function (additional endpoint) over 2-8 hours vs 54%-76% (placebo).
c. Pain relief at 2 hours (secondary endpoint) demonstrated in 61% (100 mg: 275/448) and 62% (50 mg: 547/886) of patients vs 49% (placebo: 444/912) when taken immediately or within 4 hours of onset.
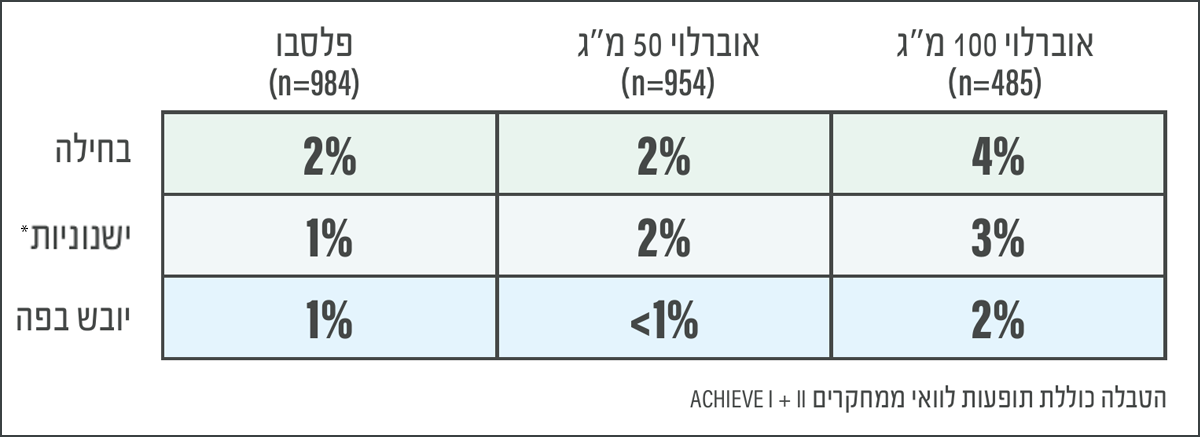
d. The most common adverse reactions were nausea (4% vs 2% placebo), somnolence (3% vs 1% placebo) and dry mouth (2% vs 1% placebo).
1. Ubrelvy prescribing information. 2. Lipton RB, et al. JAMA. 2019;322(19):1887-1898. 3. Data on file. AbbVie Inc. 4. Data on file. 5. Dodick DW, Goadsby PJ, Schwedt TJ, et al. Ubrogepant for the treatment of migraine attacks during the prodrome: a phase 3, multicentre, randomised, double-blind, placebo-controlled, crossover trial in the USA. Lancet 2023. DOI:10.1016/s0140-6736(23)01683-5.
IL-UBR-220001 | January 2026
נשמח להיות איתך בקשר!
לתיאום פגישה עם נציגה רפואית, אנא מלא/י את פרטיך בטופס הבא: