OVERVIEW
AML is an aggressive, fast-growing, molecularly, and clinically heterogeneous disease characterized by the rapid proliferation and accumulation of abnormal, immature myeloid blasts in the peripheral blood, bone marrow, and/or other tissues that can result in the suppression of normal hematopoiesis.2-4
Highly expressed BCL-2 proteins in AML cells act as gatekeepers to prevent cell death.5
INCIDENCE & MORTALITY
AML is the most common acute leukemia diagnosed in adults and the leading cause of leukemia deaths in the US and globally.
- An estimated 20,240 persons in the US will be diagnosed in 2021, and 11,400 persons will die from the disease.6
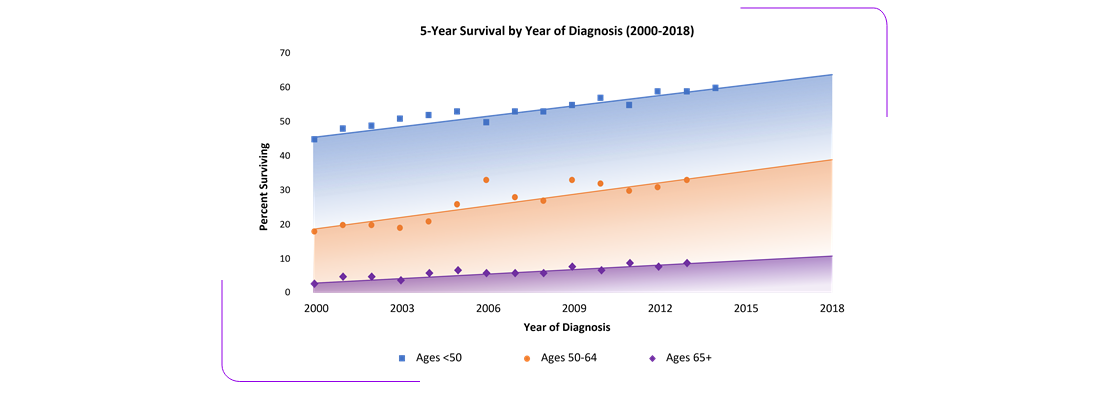
- 5-year survival rates (~30%) have not significantly improved in the past 15 years.6
AML is generally a disease of older people and is uncommon before the age of 45.7
- 85.3% of cases in the US are diagnosed in persons older than 44 years.6
- The median age at diagnosis is 68 years.6
MECHANISM OF DISEASE
AML is a clonal malignancy that can develop de novo, as a secondary malignancy (therapy-related or t-AML) following cytotoxic therapy (including alkylating agents, topoisomerase inhibitors, and antimetabolites, and/or myeloablative radio-chemotherapy), or secondary to myelodysplastic syndromes (MDS).2,8
- Both de novo and secondary AML develop through a multistep process that involves the acquisition of a variety of genetic alterations, which may induce:4,9
- A block in cell differentiation
- Increased cell proliferation
- An adverse impact on epigenetic control
- More specifically, genetic alterations and abnormalities contribute to AML pathogenesis due to their effects on tumor suppressor genes and the dysregulation of intracellular signaling pathways, apoptosis, epigenetic mechanisms, and mitochondrial metabolism.4,9,10
DIAGNOSIS & PROGNOSIS
A diagnosis of AML is made based on the presence of 20% or more blasts in the bone marrow or peripheral blood and is further defined by cytogenetic and molecular genetic changes.2
AML is a highly heterogeneous disease and expanded understanding of the molecular pathogenesis of AML has led to the identification of diagnostic and prognostic markers.2,11
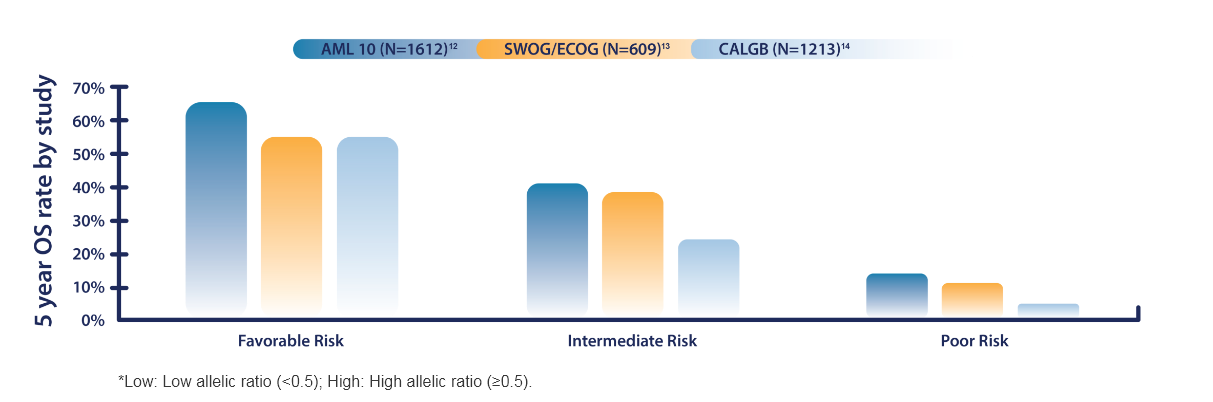
RISK STRATIFICATION BY GENETICS
Favorable
- Abnormalities of chromosome 16 at bands p13 and q22 [t(16;16)(p13;q22) or inv(16)(p13q22)]; CBFB-MYH11 fusion
- Translocation between chromosomes 8 and 21 [t(8;21)]; RUNX1-RUNX1T1 rearrangement
- Biallelic mutated CEBPA
- Mutations in the NPM1 w/o FLT3-ITD or w/FLT3-ITDlow*
Intermediate
Mutated NPM1 and FLT3-ITDhigh†
- Wild-type NPM1 w/o FLT3-ITD or w/FLT3-ITDlow† (w/o adverse risk lesions)
- Translocation between chromosomes 9 and 11 [t(9;11)] with otherwise normal chromosomes; MLLT3-KMT2A fusion
- Cytogenetic abnormalities not classified as favorable or adverse
Poor/Adverse
- Translocation between chromosomes 6 and 9 [t(6;9)]; DEK-NUP214 fusion
- Abnormalities of chromosome 11 at band q23 [t(v11q23.3); KMT2A rearranged]
- Translocation between chromosomes 9 and 22 [t(9;22)]; BCR-ABL1 fusion
- Changes to chromosome 3 at band q26 [inv(3) (q21q26.2) or t(3;3) (q21;q26.2)]
- Deletion of all or part of chromosomes 5 or 7
- Complex or monosomal karyotype
- Wild-type NPM1 and FLT3-ITDhigh*
- Mutated RUNX1, ASXL1, and TP53
CHALLENGES IN TREATMENT
Patients are stratified according to risk category by multiple factors in order to determine their ability to receive intensive induction therapy.15
The standard therapy for AML patients eligible for intensive induction therapy has not changed significantly in the past 40 years.4,9
- Induction therapy with 7+3 combination chemotherapy (7 days of cytarabine and 3 days of anthracycline).
- Followed by consolidation therapy to maintain remission (high-dose chemotherapy).
- Some eligible patients with intermediate/poor risk disease may also receive a stem cell transplant along with consolidation therapy.
Outcomes remain poor, with high rates of relapse for most patients. Older patients pose a difficult therapeutic challenge due to comorbidities and poor performance status, which may make them ineligible for intensive therapy.16
Survival in older patients who are unable to receive intensive treatment is 5-10 months.17
An increased understanding of the pathophysiology of AML has facilitated the development of novel, molecularly targeted therapies. However, these recent additions may only benefit certain patients due to the heterogeneity of the disease.
RESEARCH OPPORTUNITIES
Dramatic advances in the understanding of AML biology and genetics are being translated into strategies for targeting mutated proteins and dysregulated pathways. While these therapies address a number of areas of unmet need in AML, much clinical research and biomarker analysis remains to be done in order to expand and optimally implement these agents.18
The BCL-2 protein is overexpressed in up to 70% of AML cases; elevated levels of BCL-2 correlate with poor prognosis and chemoresistance.19,20
References
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524-548.
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
3. Leonard JP, Martin P, Roboz GJ. Practical implications of the 2016 revision of the World Health Organization classification of lymphoid and myeloid neoplasms and acute leukemia. J Clin Oncol 2017;35(23):2708-2715.
4. Cassier PA, Castets M, Belhabri A, Vey N. Targeting apoptosis in acute myeloid leukaemia. Br J Cancer. 2017;117(8):1089-1098.
5. Lauria F, et al. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia. 1997;11(12):2075-8.
6. NCI. Cancer Stat Facts: Acute Myeloid Leukemia (AML). <https://seer.cancer.gov/statfacts/html/amyl.html. Accessed November 2020>.
7. NCI. SEER*Explorer, Acute Myeloid Leukemia (AML); https://seer.cancer.gov/explorer/. Accessed September 2021.
8. Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92(10):1389-1398.
9. Kavanagh S, Murphy T, Law A, et al. Emerging therapies for acute myeloid leukemia: translating biology into the clinic. JCI Insight. 2017 Sep 21;2(18). [Epub ahead of print]
10. Lim SH, Dubielecka PM, Raghunathan VM. Molecular targeting in acute myeloid leukemia. J Transl Med. 2017;15:183. doi: 10.1186/s12967-017-1281-x.
11. Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474.
12. Grimwade D, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322-2333.
13. Slovak ML, Kopecky KJ, Cassileth PA, et al; for the Southwest Oncology Group and the Eastern Cooperative Oncology Group. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075-4083.
14. Byrd JC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13);4325-4336.
15. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
16. Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1-7.
17. Dohner H, et al. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136-52.
18. Daver N, et al. New directions for emerging therapies in acute myeloid leukemia: the next chapter. Blood Cancer J. 2020;10(10):107.
19. Campos L, Rouault J-P, Sabido O, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091-3096.
20. Mehta SV, et al. Overexpression of Bcl2 protein predicts chemoresistance in acute myeloid leukemia: its correlation with FLT3. Neoplasma. 2013;60(6):666-75.