This promotional material is intended for UK Healthcare Professionals (HCPs) experienced in the diagnosis and management of Parkinson’s disease only. Adverse event reporting can be found below
Some patients may not be suitable for PRODUODOPA. You are strongly advised to read the Prescribing information (PI), and Summary of Product Characteristics (SmPC) which can be found via the links above, to evaluate patient suitability for PRODUODOPA.
Consider PRODUODOPA for the treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson’s medicinal products have not given satisfactory results1
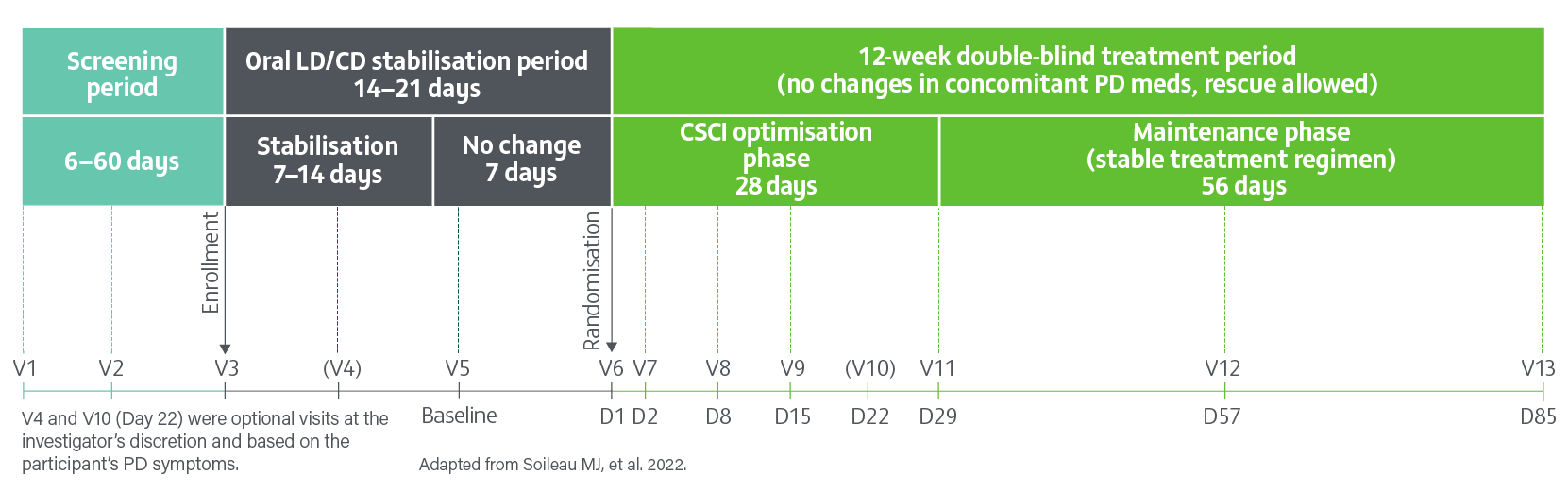
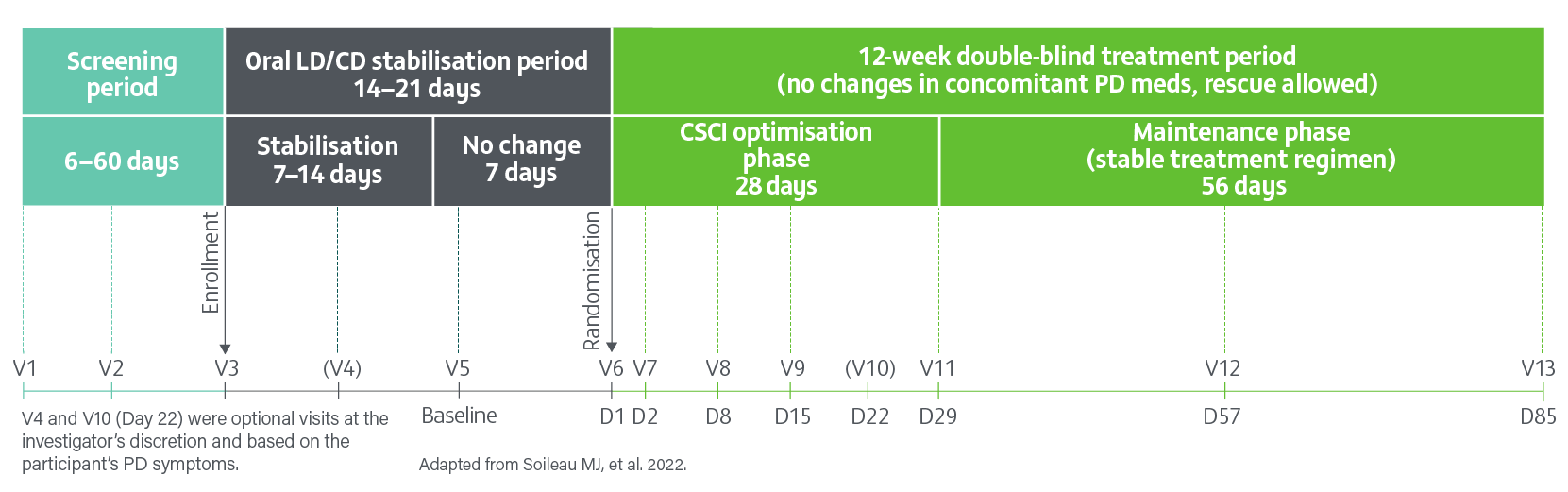
A pivotal, Phase 3, randomised, double-blind, double-dummy, active-controlled study evaluating the efficacy, safety and tolerability of 24- hour daily continuous subcutaneous infusion of PRODUODOPA (n=74) compared with oral IR levodopa/carbidopa (n=67) for the treatment of motor fluctuations in patients with advanced PD (N=141) at 12 weeks.2
- Of the 74 patients randomised to receive PRODUODOPA, 48 completed the study and 26 discontinued treatment
- Of the 67 patients receiving optimised oral IR levodopa/carbidopa (LD/CD), 62 completed treatment and 5 discontinued
- Primary reasons for drug discontinuation in both groups were adverse events, withdrawn consent, and difficulty with the drug delivery system. The incidence of adverse events leading to study drug discontinuation was higher in the PRODUODOPA group with infusion site adverse events (cellulitis 4/74 [5%]), pain (3/74 [4%]), bruising, haemmorrhage and oedema (2/74 [3%]) being most common2
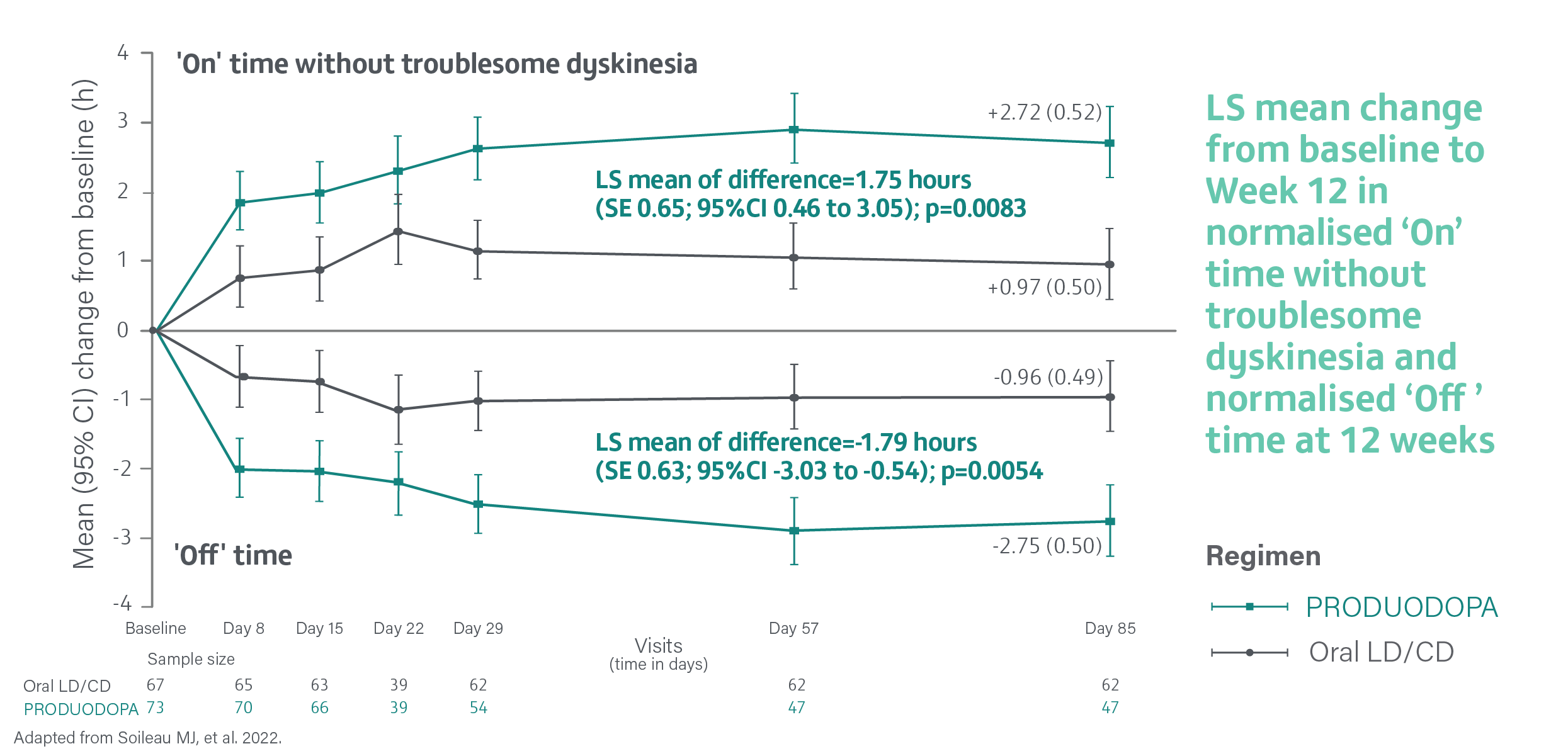
Change from baseline to Week 12 in average daily normalised ‘On’ time without troublesome dyskinesia was the primary endpoint. ‘On’ time without troublesome dyskinesia is the sum of normalised ‘On’ time without dyskinesia and normalised ‘On’ time with non-troublesome dyskinesia.2
Key secondary endpoints included change from baseline to Week 12 in average daily normalised ‘Off’ time, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part II score and presence of morning akinesia at Week 12. Morning akinesia is defined as reporting ‘Off’ status as the first morning symptom on waking.2
- Secondary efficacy endpoints were tested in a hierarchical order2
- Hierarchical testing for significance was terminated at the first secondary endpoint, change in MDS-UPDRS Part II, as this did not reach statistical significance2
- As the secondary endpoints following MDS-UPDRS Part II weren’t tested for significance, no p values are available in the referenced publication3 and no clinical conclusions can be drawn from these endpoints
‘On’ and ‘Off’ times and morning akinesia were assessed using a 24-hour Parkinson’s disease diary and normalised to a 16-hour waking day.2
In a study, PRODUODOPA provided a significant improvement (increase) in average daily normalised ‘On’ time without troublesome dyskinesia and a significant reduction (decrease) in average daily normalised ‘Off’ time vs oral IR LD/CD at 12 weeks2
Average daily normalised ‘On’ time without troublesome dyskinesia and normalised ‘Off’ time at 12 weeks2
‘On’ time without troublesome dyskinesia is the sum of normalised ‘On’ time without dyskinesia and normalised ‘On’ time with non-troublesome dyskinesia. Assessed using a 24-hour PD Diary and normalised to a 16-hour waking day. The mean daily normalised ‘On’ time without troublesome dyskinesia for patients on PRODUODOPA was 9.20 hours (SE 2.42) at baseline and 9.49 hours (SE 2.62) for patients taking optimised oral IR LD/CD. The mean daily normalised ‘Off’ time for patients on PRODUODOPA was 6.34 hours (SE 2.27) at baseline and 5.91 hours (SE 1.88) for patients taking optimised oral IR LD/CD.
Adverse events (AEs) were reported in 63 (85%) of 74 patients treated with PRODUODOPA and 42 (63%) of 67 patients treated with oral IR LD/CD at Week 122
Overview of treatment-emergent AEs (safety analysis set)2
| At 12 weeks | PRODUODOPA (n=74) | Oral LD/CD (n=67) |
Adverse events, n (%) | 63 (85) | 42 (63) |
Deaths, n (%) | 0 | 1 (1) |
Serious adverse events, n (%) | 6 (8) | 4 (6) |
Severe adverse events, n (%) | 7 (9) | 1 (1) |
Any adverse event leading to death, n (%) | 0 | 1 (1)* |
Any adverse event leading to premature study drug discontinuation,† n (%) | 16 (22) | 1 (1) |
Any adverse event considered related to study drug, n (%) | 52 (70) | 15 (22) |
Adapted from Soileau MJ, et al. 2022.
Preferred terms classified according to the Medical Dictionary for Regulatory Activities version 24.0.
*Considered by the investigator to have no reasonable possibility of being related to study drug. †Adverse events were one of the reasons for discontinuation, irrespective of whether it was the primary reason.
AEs of special interest2
| At 12 weeks | PRODUODOPA (n=74) | Oral LD/CD (n=67) |
Infusion site events, n (%) | 53 (72) | 8 (12) |
Hallucinations or psychosis, n (%) | 11 (15) | 2 (3) |
Falls and associated injuries, n (%) | 13 (18) | 17 (25) |
Somnolence, n (%) | 1 (1) | 1 (1) |
Polyneuropathy, n (%) | 2 (3) | 2 (3) |
Weight loss, n (%) | 1 (1) | 1 (1) |
Adapted from Soileau MJ, et al. 2022.
Most frequent (≥5%) AEs2
| At 12 weeks | PRODUODOPA (n=74) | Oral LD/CD (n=67) |
Infusion site erythema, n (%) | 20 (27) | 1 (1) |
Infusion site pain, n (%) | 19 (26) | 1 (1) |
Infusion site cellulitis, n (%) | 14 (19) | 0 |
Infusion site oedema, n (%) | 9 (12) | 0 |
Dyskinesia, n (%) | 8 (11) | 4 (6) |
Fall, n (%) | 6 (8) | 12 (18) |
Infusion site bruising, n (%) | 6 (8) | 2 (3) |
Infusion site haemorrhage, n (%) | 6 (8) | 0 |
Infusion site nodule, n (%) | 6 (8) | 0 |
‘On’ and ‘Off’ phenomena, n (%) | 6 (8) | 0 |
Hallucination, n (%) | 5 (7) | 1 (1) |
Balance disorder, n (%) | 4 (5) | 0 |
Constipation, n (%) | 4 (5) | 0 |
Hallucination visual, n (%) | 4 (5) | 0 |
Infusion site induration, n (%) | 4 (5) | 0 |
Infusion site infection, n (%) | 4 (5) | 0 |
Infusion site pruritus, n (%) | 4 (5) | 0 |
Peripheral swelling, n (%) | 4 (5) | 0 |
Adapted from Soileau MJ, et al. 2022.
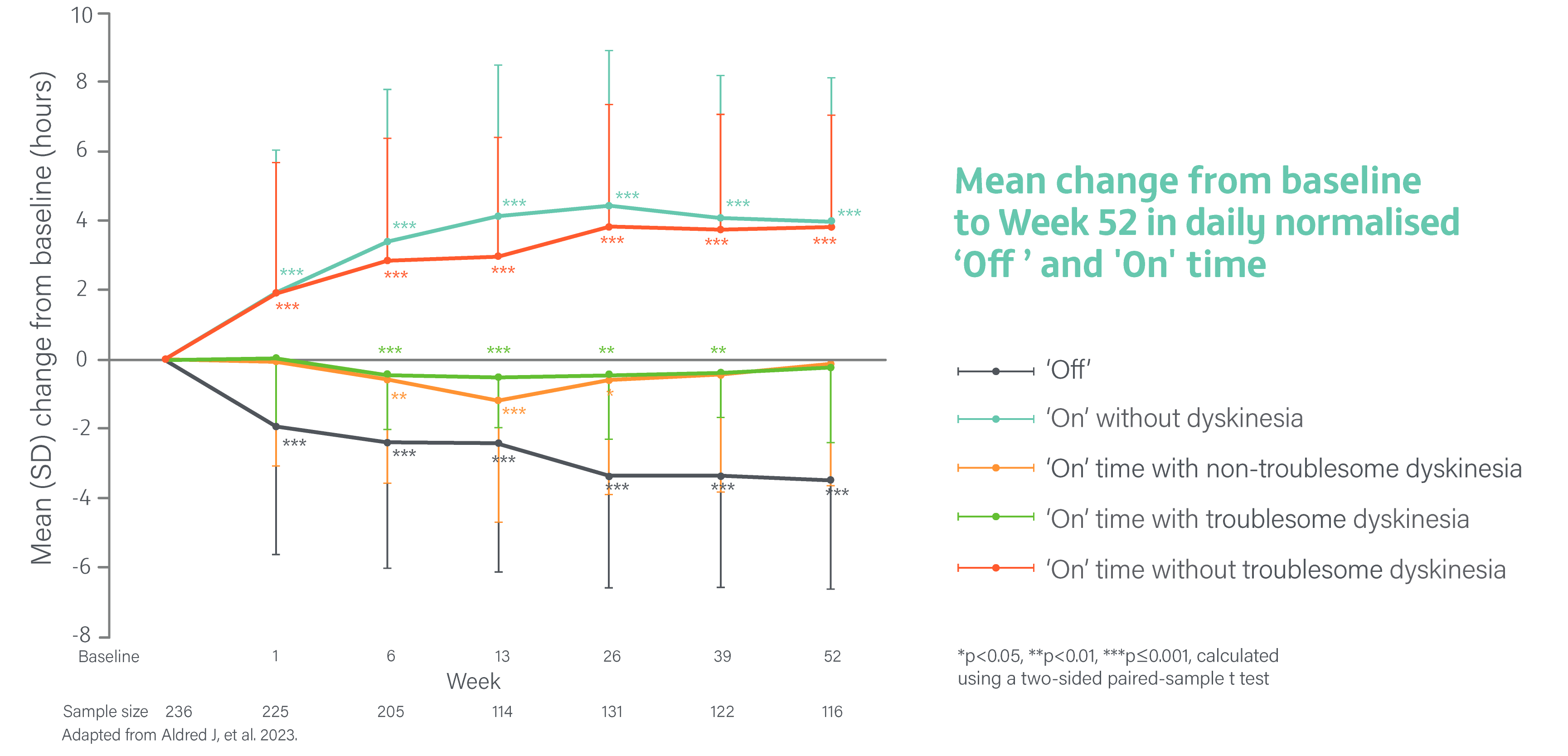
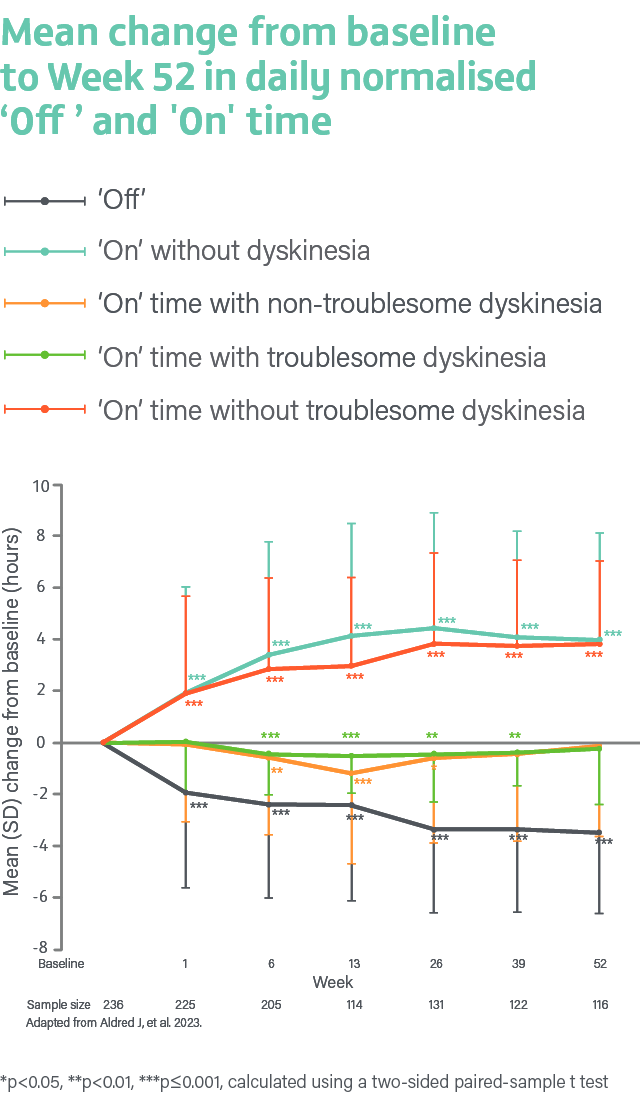
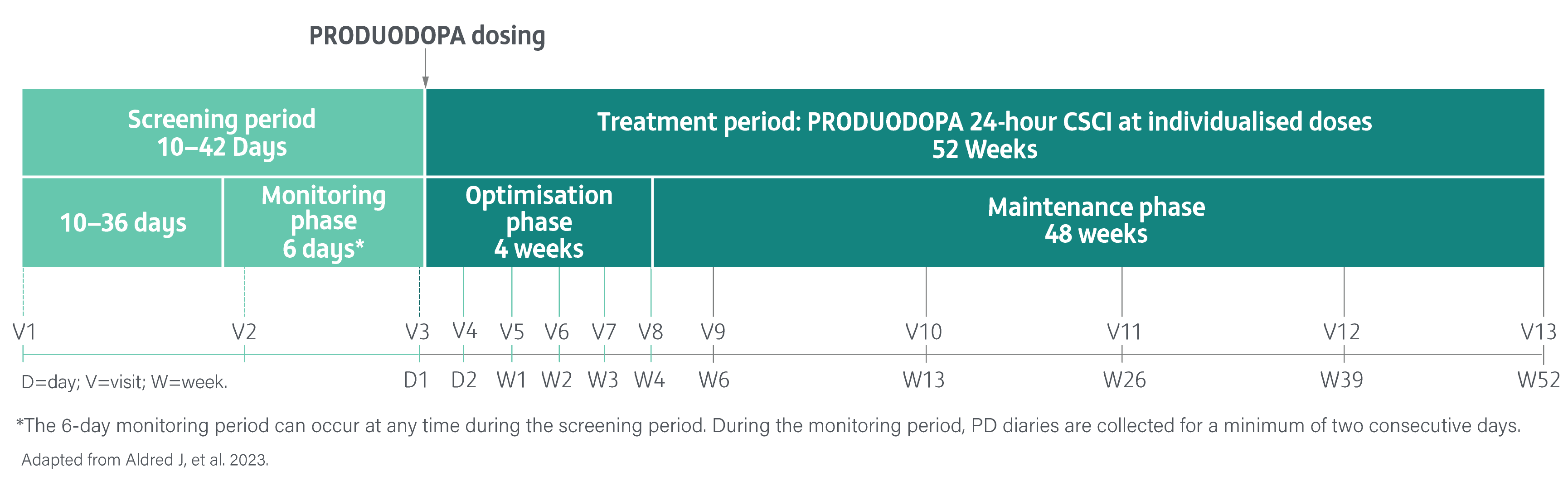
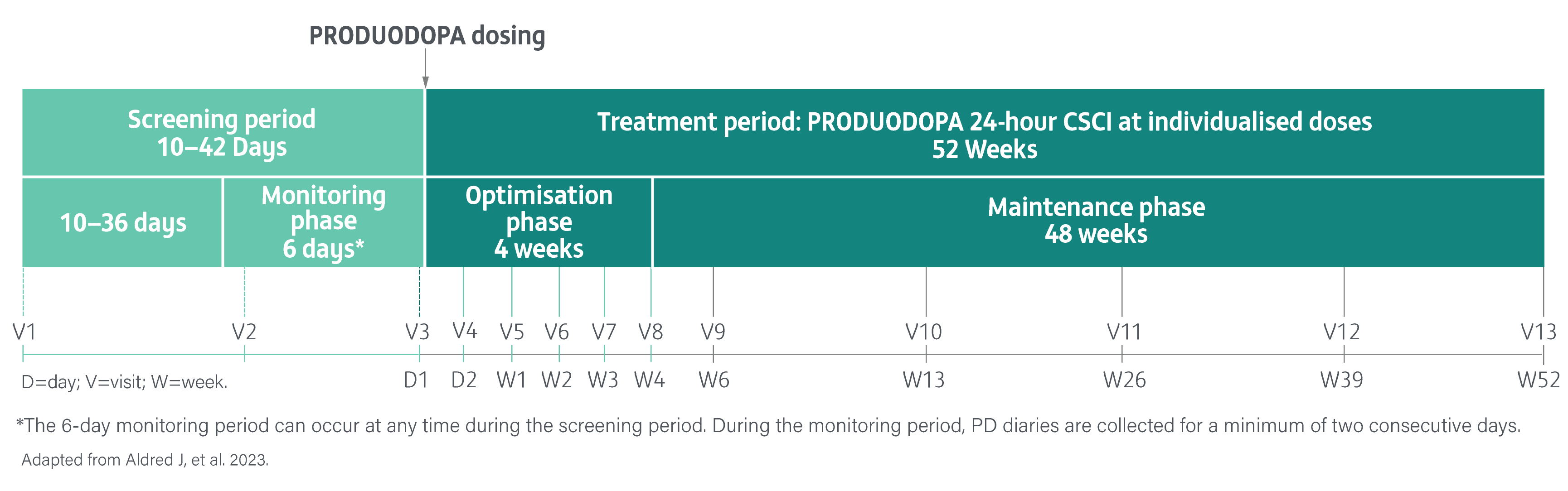
A Phase 3, single-arm, open-label study evaluating the safety, tolerability, and efficacy of 24-hour daily exposure with a continuous subcutaneous infusion of PRODUODOPA in patients with advanced PD (N=244) at 52 weeks. Of 244 enrolled patients, 107 (44%) discontinued treatment and 137 completed treatment with PRODUODOPA.3
Safety and tolerability were the primary endpoints. The proportion of patients reporting adverse events, changes in laboratory parameters, vital sign measurements, and electrocardiogram values from baseline. Local skin tolerability was additionally assessed by the Infusion Site evaluation scale.3
Secondary endpoints included change from baseline in the average daily normalised ‘Off’ time and ‘On’ time as assessed by the PD diary and normalised to a 16-hour waking day, MDS-UPDRS Parts I-IV (with Part III measured in the patient’s best on state), Parkinson’s Disease Sleep Scale-2 (PDSS-2), 39-Item Parkinson’s Disease Questionnaire (PDQ-39), EuroQol 5-Dimension Questionnaire (EQ-5D-5L) and the proportion of patients with morning akinesia. Morning akinesia was defined as reporting ‘Off’ status in PD diary as the predominant PD status during the first half-hour period upon awakening.3
The safety analysis set included all patients who received at least one infusion of PRODUODOPA at any given time during the study. Efficacy was assessed in the full analysis set.
Overview of treatment-emergent adverse events (safety analysis set)3
| At 52 weeks | PRODUODOPA (N=244) |
AEs, n (%) | 230 (94.3) |
AEs associated with study drug, n (%) | 224 (91.8) |
Severe AEs,* n (%) | 63 (25.8) |
Serious AEs,* n (%) | 63 (25.8) |
AEs leading to discontinuation of study drug, n (%) | 64 (26.2) |
Deaths,†‡ n (%) | 3 (1.2) |
Adapted from Aldred J, et al. 2023.
Preferred terms classified using the Medical Dictionary for Regulatory Activities, version 24.0.
*While the values for severe AEs and serious AEs were the same, the patients reporting these events were not the same for each event. †There were a total of five deaths in the safety population; two were non-treatment-emergent (did not occur during study treatment or within 30 days of the last PRODUODOPA infusion); all were determined by investigators to be unrelated to the study drug. ‡Treatment-emergent deaths include subdural hematoma (most likely related to traumatic injury from an accidental fall) and cerebral mass effect, cardiorespiratory arrest, and cerebrovascular accident.
AEs of special interest3
| At 52 weeks | PRODUODOPA (N=244) |
Infusion site reactions, n (%) | 200 (82.0) |
Infusion site-related infections, n (%) | 86 (35.2) |
Falls and associated injuries, n (%) | 74 (30.3) |
Hallucinations/psychosis, n (%) | 61 (25.0) |
Weight loss, n (%) | 27 (11.1) |
Somnolence, n (%) | 12 (4.9) |
Polyneuropathy (narrow SMQ), n (%) | 8 (3.3) |
Adapted from Aldred J, et al. 2023.
Preferred terms classified using the Standardized Medical Dictionary for Regulatory Activities query (SMQ).
Most frequent (>10%) AEs3
| At 52 weeks | PRODUODOPA (N=244) |
Infusion site erythema, n (%) | 127 (52.0) |
Infusion site nodule, n (%) | 70 (28.7) |
Infusion site cellulitis, n (%) | 56 (23.0) |
Infusion site oedema, n (%) | 47 (19.3) |
Hallucination, n (%) | 42 (17.2) |
Fall, n (%) | 41 (16.8) |
Infusion site pain, n (%) | 38 (15.6) |
Infusion site reaction, n (%) | 30 (12.3) |
Anxiety, n (%) | 29 (11.9) |
Infusion site abscess, n (%) | 27 (11.1) |
Dizziness, n (%) | 25 (10.2) |
Adapted from Aldred J, et al. 2023.
SAEs occurring in >2 patients3
| At 52 weeks | PRODUODOPA (N=244) |
Infusion site cellulitis, n (%) | 10 (4.1) |
Infusion site abscess, n (%) | 8 (3.3) |
Hallucination, n (%) | 7 (2.9) |
Parkinson’s disease, n (%) | 6 (2.5) |
Psychotic disorder, n (%) | 6 (2.5) |
Urinary tract infection, n (%) | 4 (1.6) |
Sepsis, n (%) | 3 (1.2) |
Pneumonia, n (%) | 3 (1.2) |
Adapted from Aldred J, et al. 2023.
Efficacy endponts3
Efficacy endpoints were summarised using descriptive statistics. P values throughout this study are nominal and therefore no clinical conclusions can be drawn from this data.3
Average daily normalised ‘Off’ time and ‘On’ time
The mean daily normalised ‘Off’ time for patients on PRODUODOPA was 5.9 hours (SD 2.2) at baseline. The mean daily normalised ‘On’ time without dyskinesia was 6.5 hours (SD 3.4) at baseline. The mean daily normalised ‘On’ time with non-troublesome dyskinesia was 2.6 hours (SD 2.6) at baseline. The mean daily normalised ‘On’ time with troublesome dyskinesia was 1.0 hours (SD 1.7) at baseline. The mean daily normalised ‘On’ time without troublesome dyskinesia was 9.1 hours (SD 2.5) at baseline.
CI=confidence interval; EQ-5D-5L=EuroQol 5-Dimension Questionnaire; IR=immediate release; LD/CD=levodopa/carbidopa; LS=least squares; MDS-UPDRS=Movement Disorder Society-Unified Parkinson’s Disease Rating Scale; PDQ-39=39-Item Parkinson’s Disease Questionnaire; PD=Parkinson’s disease; PDSS-2=Parkinson’s Disease Sleep Scale-2; SD=standard deviation; SE=standard error; SMQ=Standardized Medical Dictionary for Regulatory Activities query.
Please refer to the PRODUODOPA Summary of Product Characteristics (SmPC) for further information on adverse events, contraindications and special warnings and precautions for use.
References
- PRODUODOPA (foslevodopa/foscarbidopa solution for infusion) Summary of Product Characteristics.
- Soileau MJ, et al. Lancet Neurol. 2022;21:1099–1109.
- Aldred J, et al. Neurol Ther. 2023 Dec;12(6):1937-1958. doi: 10.1007/5. s40120-023-00533-1.
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
UK-PRODD-240199. Date of preparation: December 2024.