Preventative treatment plans should consider the individual needs of patients with migraine2
According to the 2019 British Association for the Study of Headache guidelines, patients who experience ≥4 migraine days/month should be offered preventative treatment as an option, as this frequency of migraine is associated with significant disability.2
Prescribing decisions should take into account a patient’s current clinical situation as well as their future plans2
When choosing a preventative treatment, factors to consider include:2
Previous treatment
Personal preferences
Medical and other
comorbidities
Side effect profiles of various treatments
Pregnancy or contraception
AQUIPTA® is not recommended during pregnancy.1
Introducing AQUIPTA®: an oral, once‑daily CGRP receptor antagonist for migraine prevention1,3
AQUIPTA® is indicated for prophylaxis of migraine in adults who have at least 4 migraine days/month. It is a selective CGRP receptor antagonist that blocks the binding of CGRP to the receptor and antagonises CGRP receptor function. By blocking the CGRP‑receptor interaction, AQUIPTA® prevents migraine attacks.1
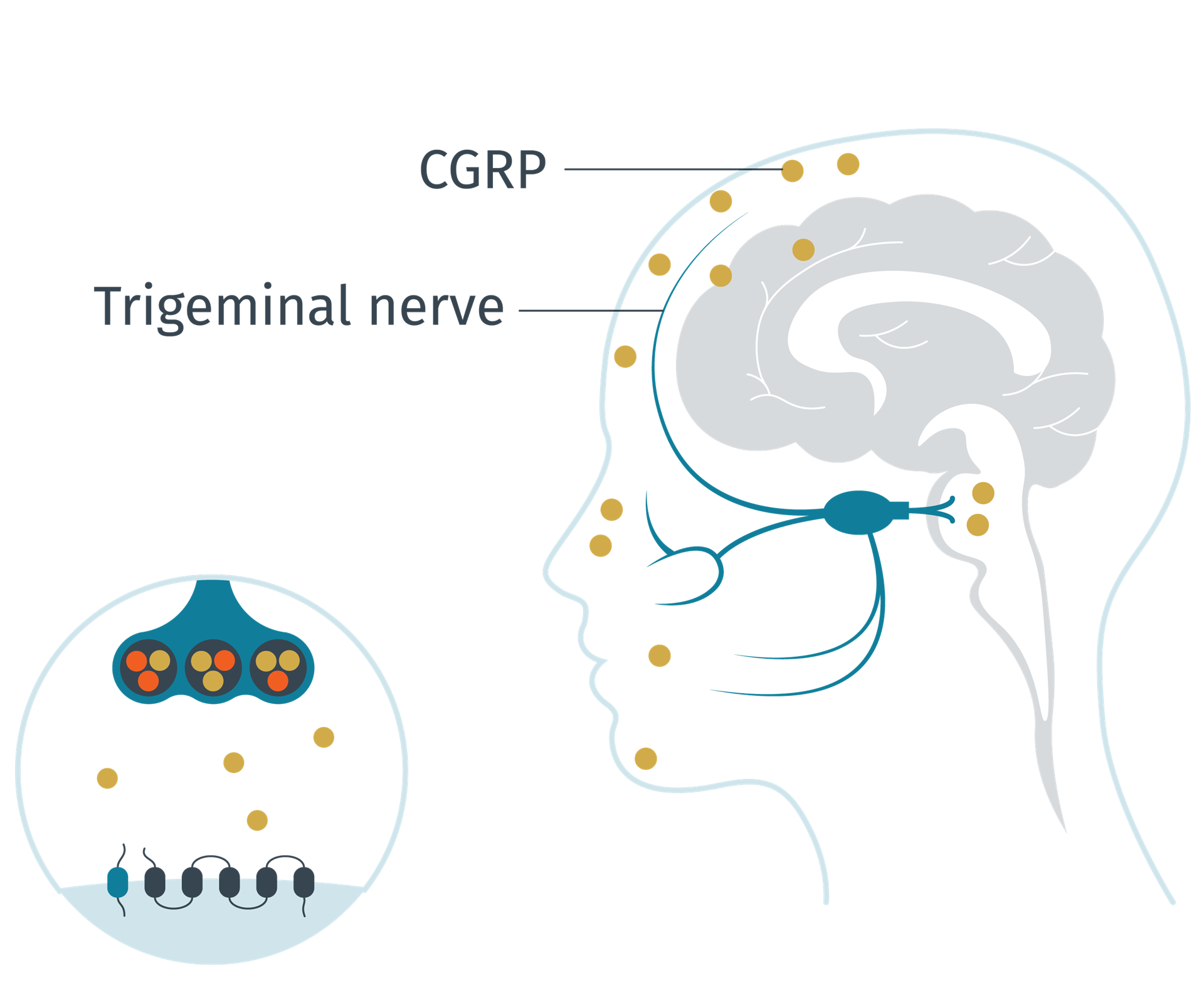
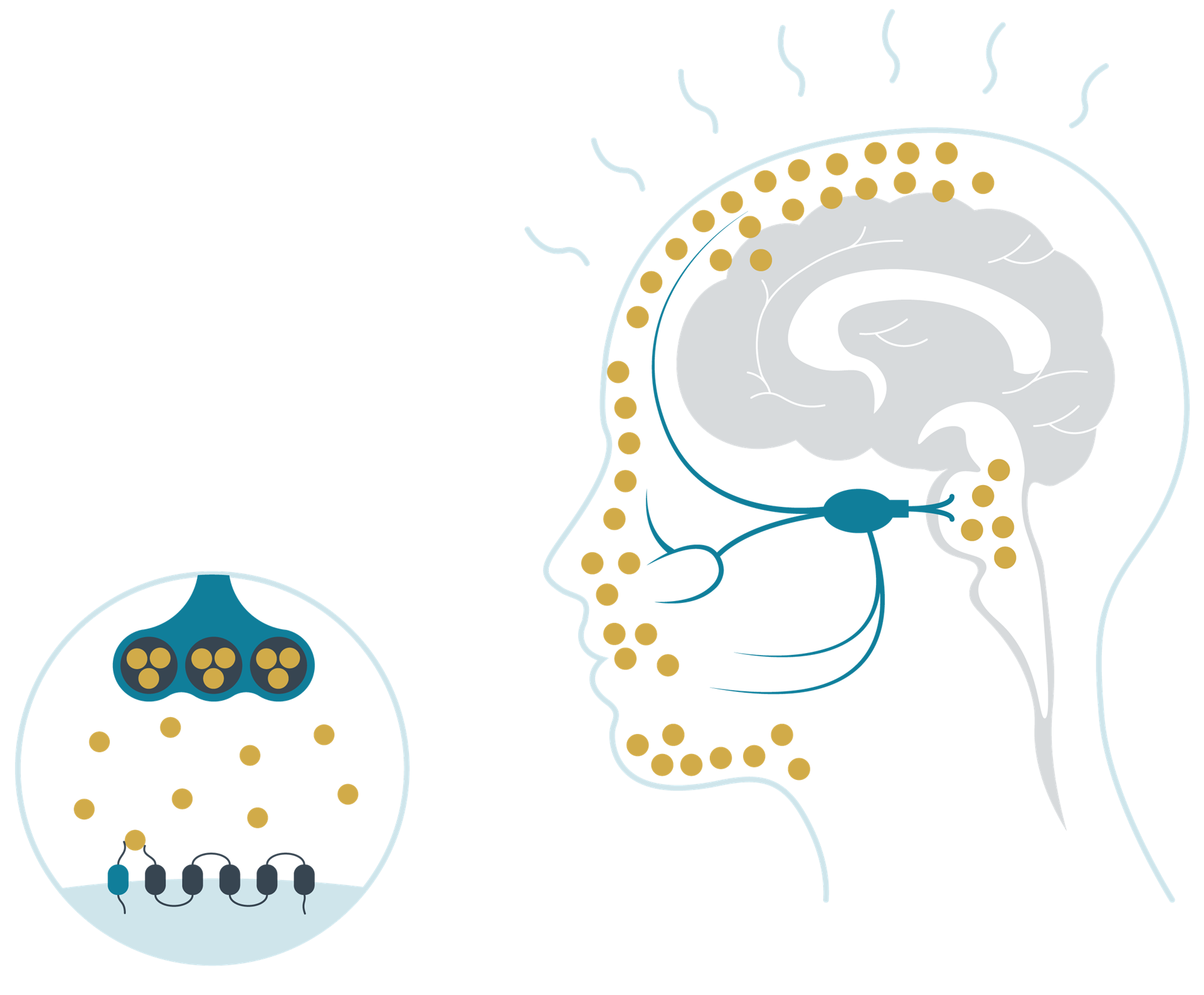
CGRP levels during a migraine attack
Adapted from Schuster N M et al. 20164
During a migraine attack, levels of the neuropeptide increase.5,6 In the trigeminovascular system, CGRP modulates nociceptive signalling and inflammation, and also functions as a vasodilator,1 inducing pain, inflammation and vasodilation.7
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE (NICE) GUIDANCE8
AQUIPTA® is recommended by NICE as an option for preventing migraine in adults who have at least 4 migraine days per month, only if at least 3 preventive medicines have not worked, or are not tolerated or are unsuitable because of safety concerns.8
Stop AQUIPTA® after 12 weeks if the frequency of migraines does not reduce by:8
> At least 50% in episodic migraine (defined as fewer than 15 headache days per month)
> At least 30% in chronic migraine (defined as 15 or more headache days per month, with at least 8 of those having features of migraine)
SCOTTISH MEDICINES CONSORTIUM
(SMC) GUIDANCE9
AQUIPTA® is accepted by SMC for restricted use within NHS Scotland for the prophylaxis of migraine in adults who have at least 4 migraine days per month.9
SMC restriction: for patients with chronic and episodic migraine who have had prior failure on three or more migraine preventative treatments.9
AQUIPTA® provides an additional treatment choice in the therapeutic class of CGRP inhibitors.9
WANT TO KNOW MORE ABOUT THE EFFICACY OF AQUIPTA® IN SPECIFIC TYPES OF MIGRAINE?
CGRP: calcitonin gene-related peptide; NICE: National Institute for Health and Care Excellence; SMC: Scottish Medicines Consortium.
References:
- AQUIPTA® Summary of Product Characteristics.
- British Association for the Study of Headache (BASH). National headache management system for adults 2019. Available at: https://bash.org.uk/wp-content/uploads/brizy/custom_files/01_BASHNationalHeadache_Management_SystemforAdults_2019_guideline_versi.pdf. Accessed November 2025
- Moreno-Ajona D, Villar-Martínez M D, Goadsby P J. New generation gepants: migraine acute and preventive medications. J Clin Med 2022;11(6):1656
- Schuster N M, Rapoport A M. New strategies for the treatment and prevention of primary headache disorders. Nat Rev Neurol. 2016;12(11):635–650
- Rivera-Mancilla E, Villalón C M, Maassen van den Brink A. CGRP inhibitors for migraine prophylaxis: a safety review. Expert Opin Drug Saf. 2020;19(10):1237–1250
- Ferrari M D, Goadsby P J et al. Migraine. Nat Rev Dis Primers 2022;8(1):2
- Goadsby P J, Holland P R et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance 973. Atogepant for preventing migraine, 15 May 2024. Available at: https://www.nice.org.uk/guidance/ta973. Accessed November 2025
- Scottish Medicines Consortium (SMC). SMC2599 Atogepant (Aquipta). Available at: https://scottishmedicines.org.uk/medicines-advice/atogepant-aquipta-abbreviated-smc2599/. Accessed November 2025
Please refer to the AQUIPTA® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The AQUIPTA® Summary of Product Characteristics can be found here.
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
UK-AQP-250451 | Date of preparation: November 2025.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk
Adverse events should also be reported to AbbVie on GBPV@abbvie.com



.png)