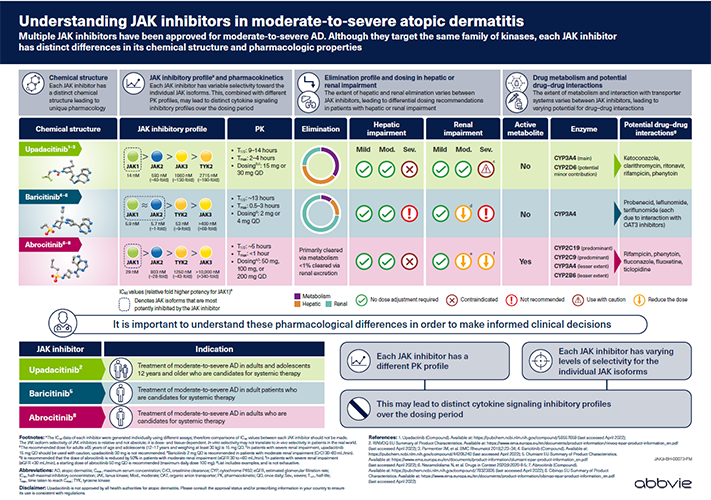
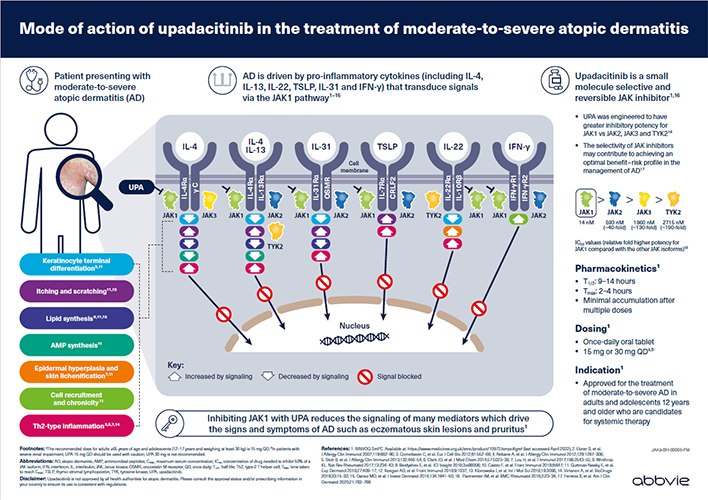
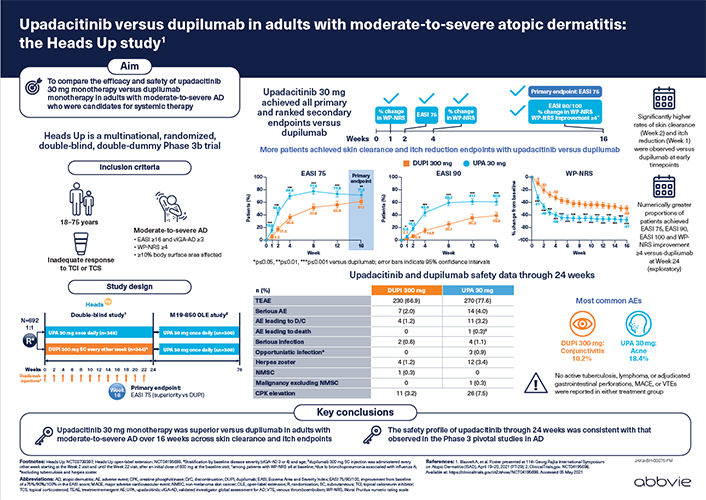
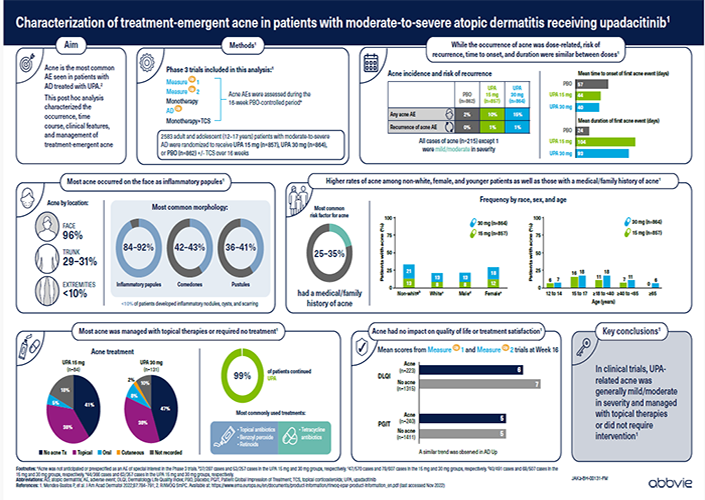
For Oman HCPs.
Department of Pharmacovigilance & Drug information.
Directorate General of pharmaceutical affairs and Drug control.
Ministry of health, Sultanate of Oman
Phone Nos:: 22357687 / 22357686
Fax: 22358489
Email: Pharma-Vigil@moh.gov.om
Website:www.moh.gov.om
This website is intended for Gulf Healthcare Professionals
AbbVie Biopharmaceuticals GmbH.
Suite 2901, U Bora Tower,Business Bay, Dubai, UAE
PO Box:: 118052
OFFICE : +971 4 5506100
FAX: +971 4 5506101
Patient Safety is our Priority, we will remain always by your side. For Reporting Adverse events Please Contact.
PV.MEA@Abbvie.com or Hotline: 00971564135746
JAKa-BH-00004-MC