How to diagnose
Headache, including migraine, is one of the top 10 reasons for visiting a GP, with consultation rates in the UK of 6.4 per 100 female adult patients per year and 2.5 per 100 male adult patients.6 25% of new referrals to neurologists are for headache.7
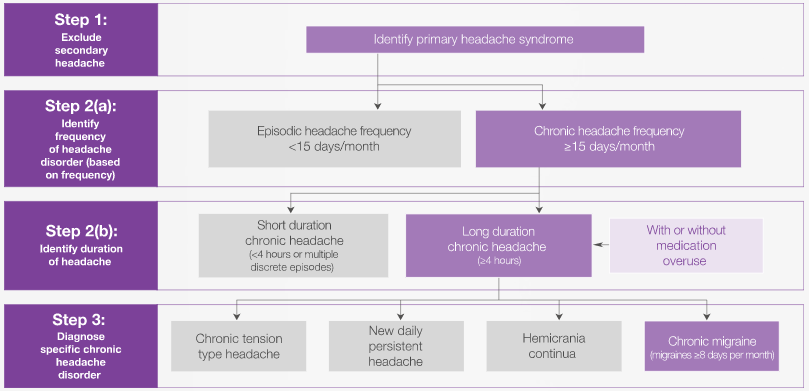
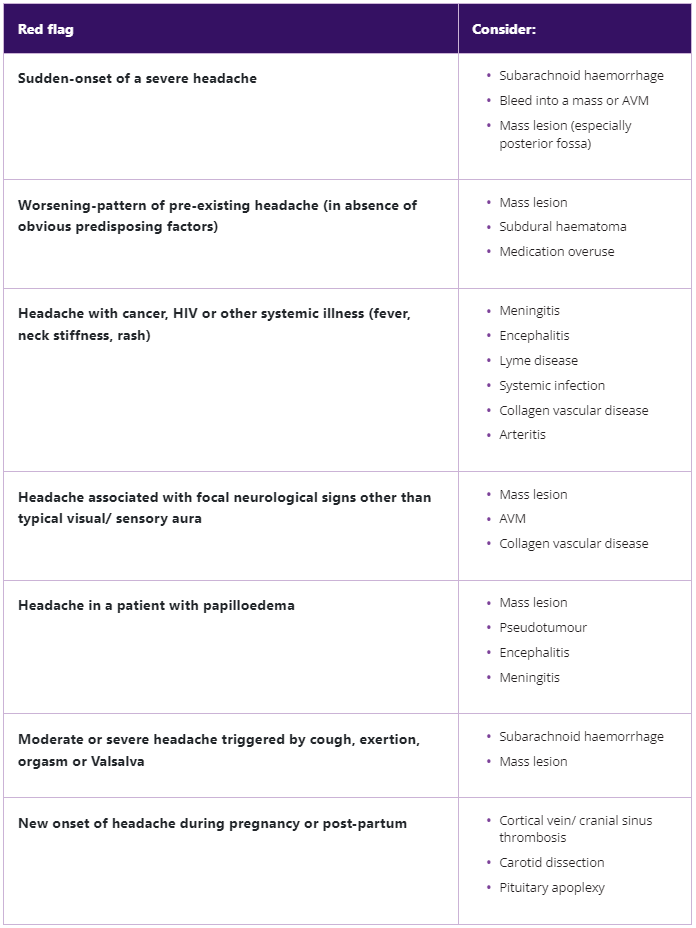
It can be challenging to accurately diagnose patients with chronic migraine.8 In one study of 512 patients who met the criteria for chronic migraine and were consulting a healthcare professional, only 24.6% had received an accurate diagnosis.8 The first diagnostic step is to distinguish a primary headache from a secondary headache; secondary headaches can be indicated by ‘red flag’ symptoms and will likely require specific tests for confirmation of diagnosis.9
Having excluded secondary headaches, the next step is to diagnose a specific primary headache syndrome, based on frequency and duration of headache.9-11
Listen to Dr Andrew Blumenfeld, Director of the Headache Center of Southern California, US, discuss the unmet need for better diagnosis in chronic migraine, differential diagnoses and chronic daily headache classification.
Risk factors for developing chronic migraine
Chronic migraine often develops from episodic migraine after a period of increasing headache frequency. This higher frequency is associated with a number of risk factors, including:11,12
- age
- sex (female)
- obesity
- snoring
- head injury
- stressful life events
- depression and/or anxiety
- cutaneous allodynia
- overuse of opioids
- overuse of barbiturates
- high caffeine intake
- ineffective acute headache treatment
Chronic migraine is a complex neurological disease requiring appropriate effective management - including treatment or referrals to improve patient outcomes2,10,13
CM: chronic migraine.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: March 2024. UK-BCM-240037