UK-RISN-230338. Date of preparation January 2024.
Safety Profile in Psoriasis
Learn more about the safety profile of SKYRIZI in clinical trials and long-term studies for psoriasis and psoriatic arthritis.
*Contraindications include hypersensitivity to the active substance or to any of the excipients and clinically important active infections e.g. active tuberculosis.
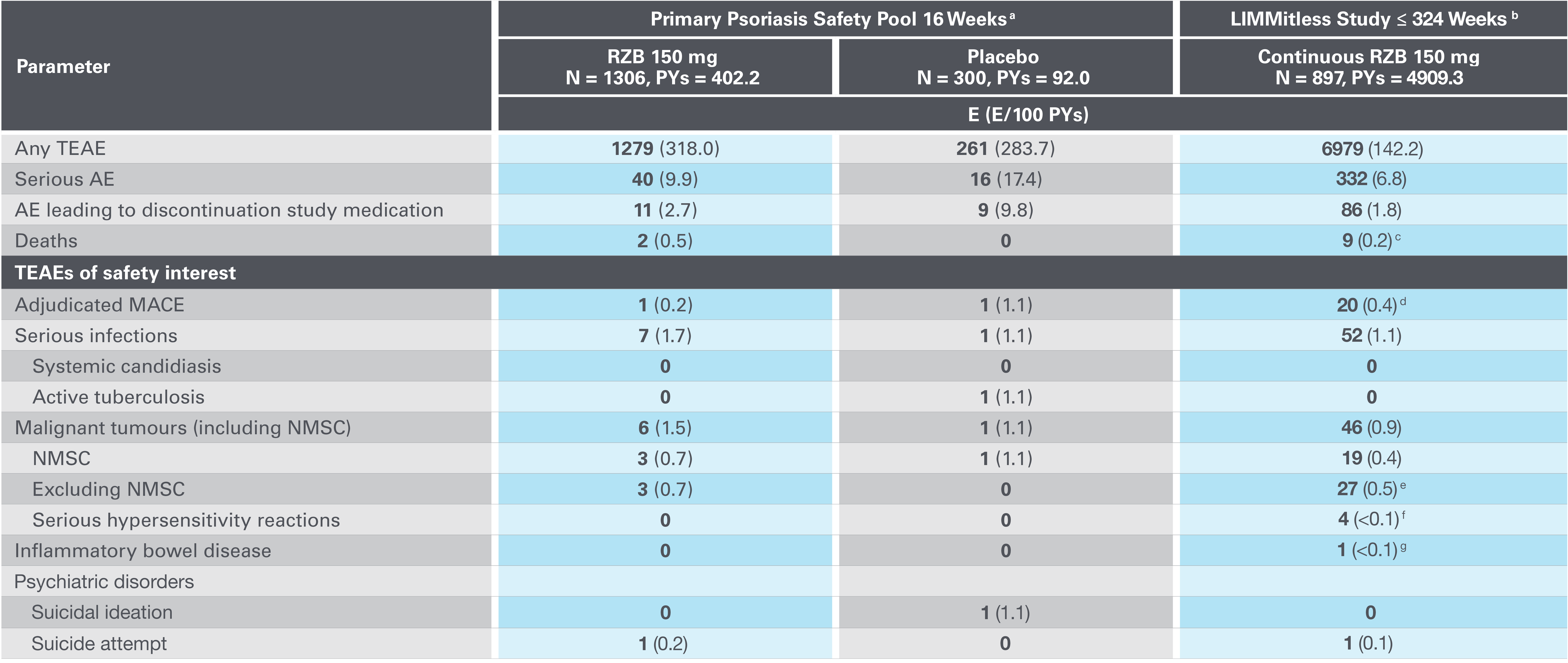
Adapted from Papp K, et al. 2023.
AE, adverse event; E, events; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PYs, patient-years; RZB, risankizumab; TEAE, treatment-emergent AE.
aPrimary psoriasis safety pool includes UltIMMa-1, UltMMa-2, IMMhance, IMMvent and NCT0205448110 studies.
b16-week safety data for patients who received RZB 150 mg UltIMMa-1, UltMMa-2, and IMMvent (primary psoriasis safety pool) are included in the 324-week LIMMitless results.
cDue to natural causes (n=1), accident (n=1), cardiovascular event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=2), and COVID-19 infection (n=1); none related to RZB.
dMACE rate in the LIMMitless study is consistent with the incidence rate of MACE in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65).
eMalignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1).
fSerious hypersensitivity reactions (all of which were considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), and Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]).
gOne nonserious event of ulcerative colitis, considered unrelated to RZB.
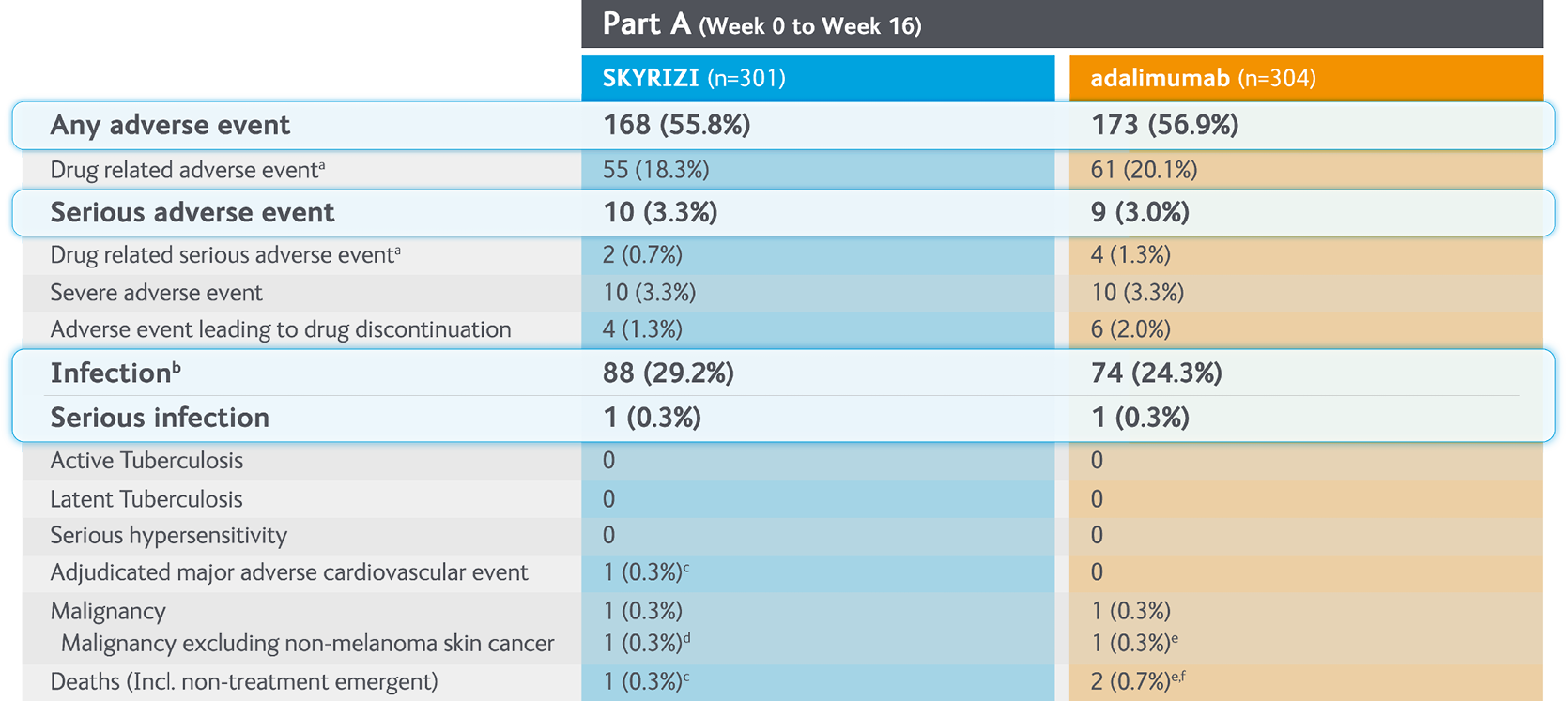
Summary of treatment-emergent adverse events with SKYRIZI vs adalimumab in Part A (Weeks 0-16) of the IMMvent Phase III clinical trial6
Randomised controlled trial, intention to treat population (Part A).
aInvestigator assessed AE as possibly related to study drug.
bThe most frequently reported infectious AE were viral upper respiratory tract infection and upper resporatory tract infarction.
cOne patient with acute myocardial infarction on study day 73 (event was not considered to be study grud related by investigator).
dOne patient diagnosed with invasive lobular breast carcinoma on study day 63 following routine mammogram (event was not considered to be study drug related by investigator).
eOne patient with stage IV gall bladder cancer.
fOne patient with cholelithiasis, underwent gall bladder surgery, developed cardiopulmonary arrest and died due to abdominal abscess, sepsis, and gastric perforation (events were not considered to be study drug related by investigator).
AE, adverse event; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer.
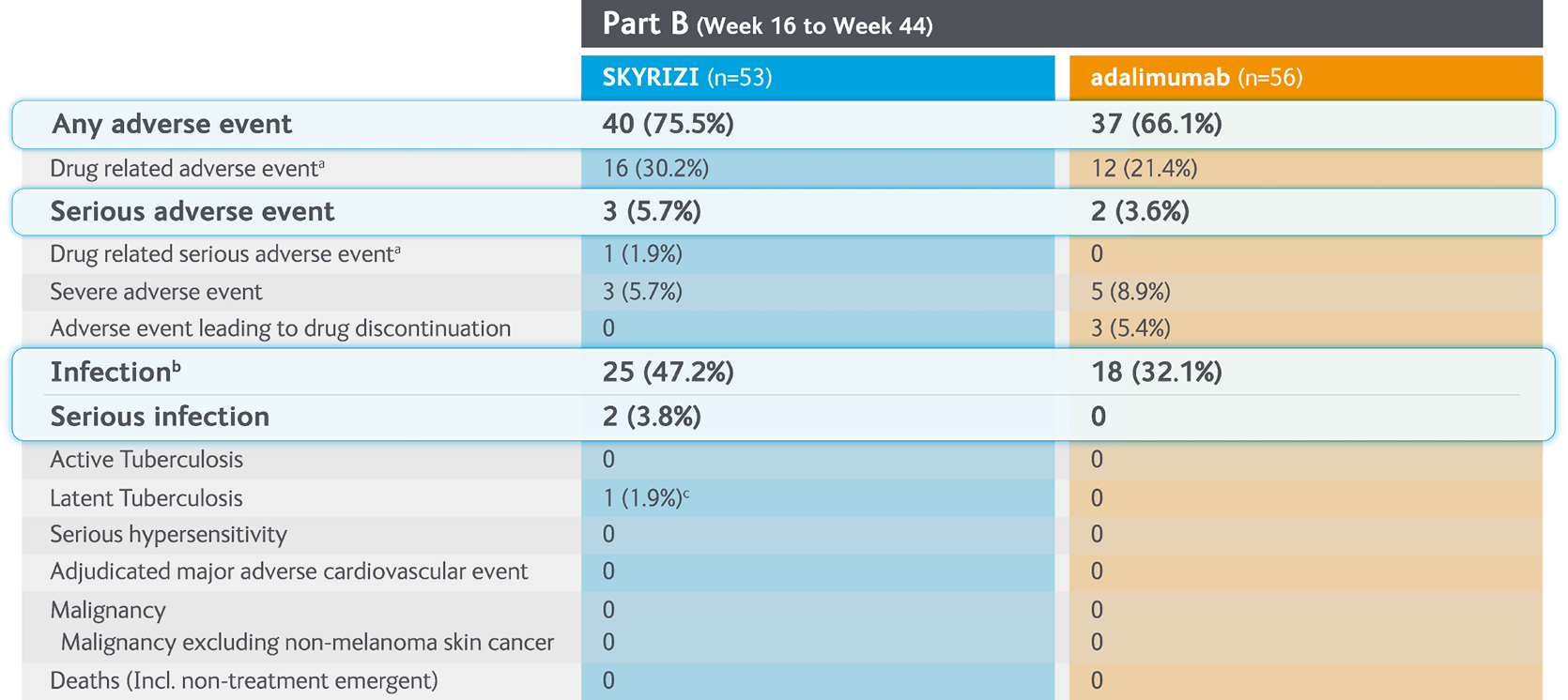
Summary of treatment-emergent adverse events with SKYRIZI vs. adalimumab in Part B (Weeks 16-44) of the IMMvent Phase III clinical trial6
Randomised controlled trial. lntention to population (Part B). Patients previously treated with adailmumab. randomised to adallrnumab or SKYRIZI at Week 16.
aInvestigator assessed AE as possibly related to study drug.
bThe most frequently reported Infectious AE were viral upper respiratory tract Infection and upper respiratory tract Infection.
cOne patient with latent tuberculosis. positive TB test during the study.
AE, adverse event; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer.
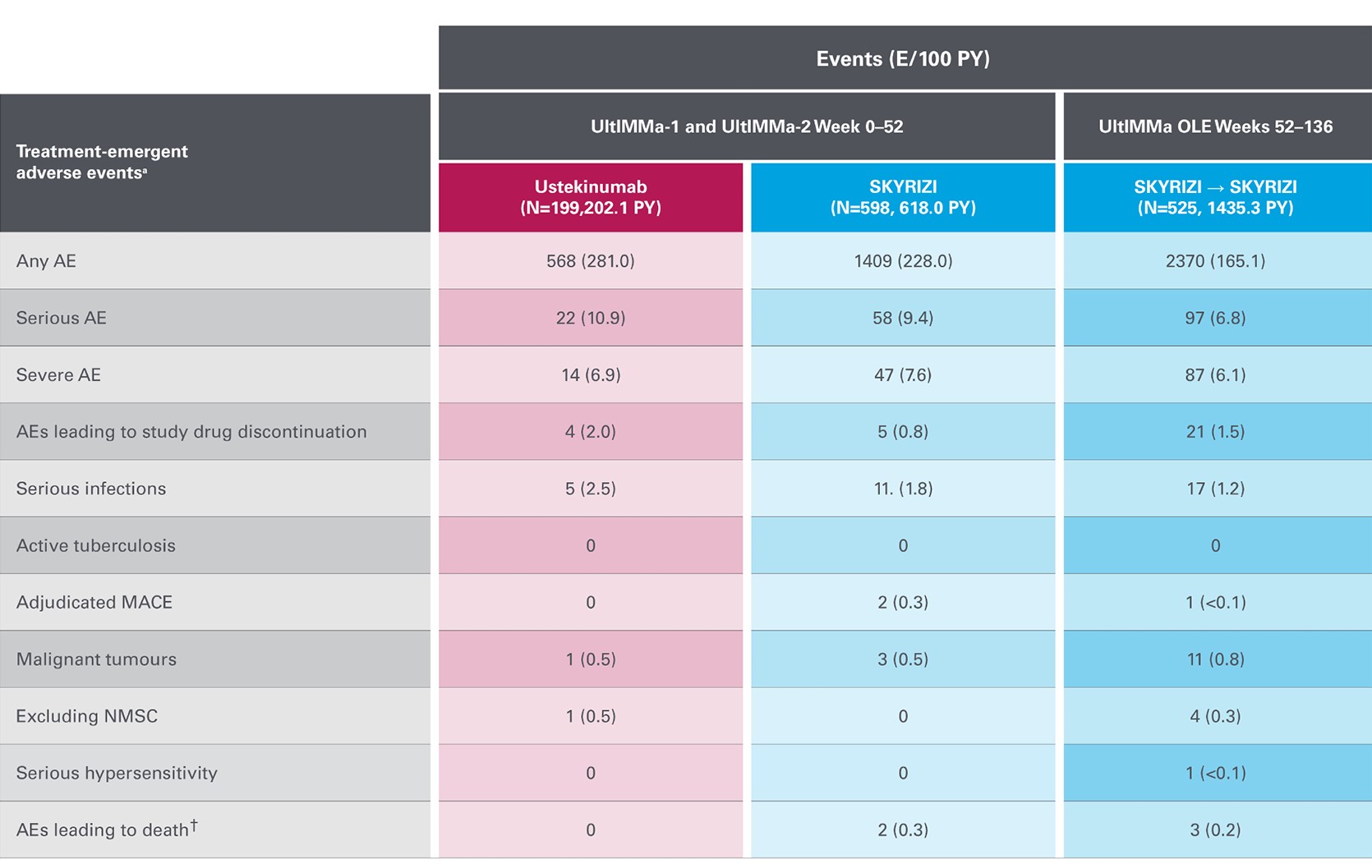
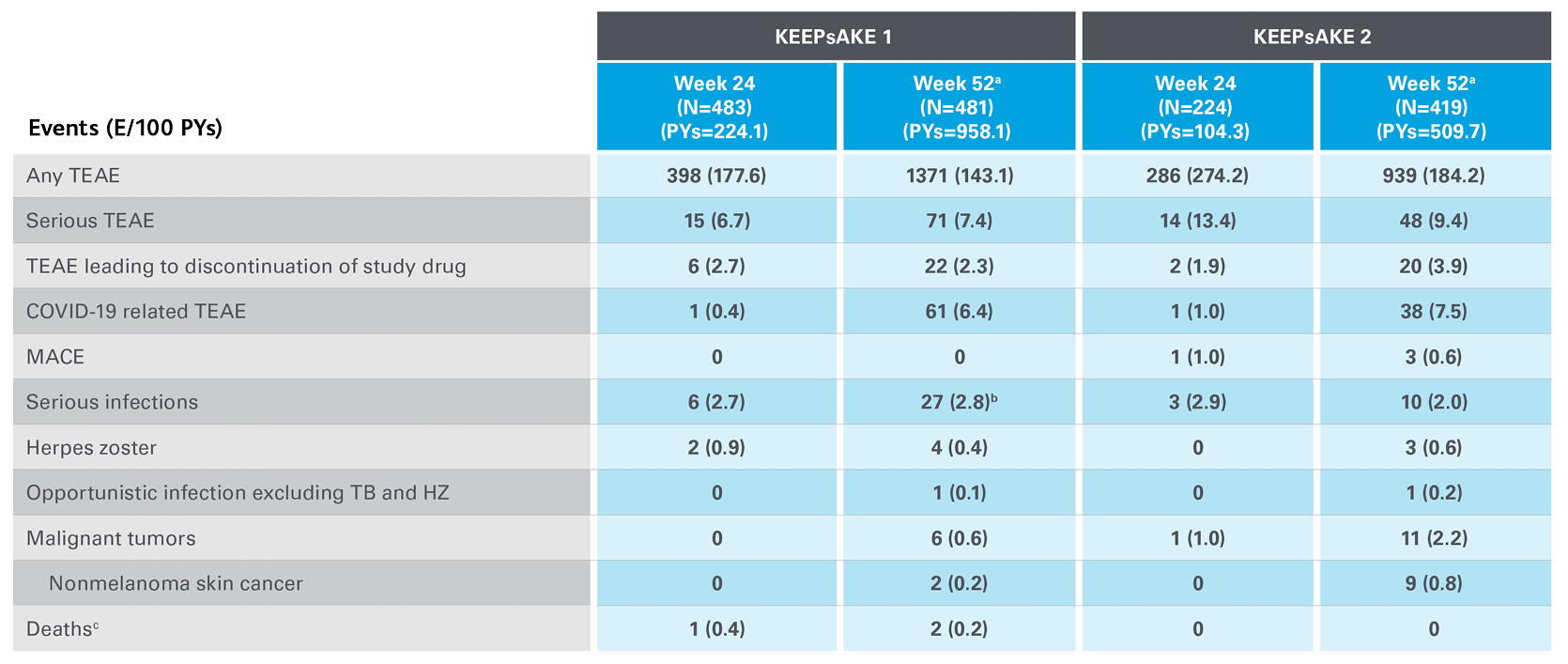
Safety events from KEEPsAKE 1 and KEEPsAKE 2 through to week 528
Adapted from Kristensen LE, et al. 2021.
aSafety reported through data cutoff date (19 April 2021), which includes data through week 52. Data are from any RZB 150-mg group that includes all patients who received RZB 150 mg, including those who started on RZB 150 mg at randomisation and who switched from placebo to RZB 150 mg after week 24.
b10 of the 27 events were cases of COVID-19.
cAn 81-year-old male patient randomised to RZB died of urosepsis on day 96, and a 41-year-old male patient randomised to RZB experienced sudden death on day 502.
E, events; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer; PY, patient-years; RZB, risankizumab; TB, tuberculosis; TEAE, treatment-emergent adverse events.
Featured content
UK-RISN-230325. Date of preparation February 2024.
References
- Reich K, et al. Lancet 2019; 394: 576-586.
- Gordon KB, et al. Lancet 2018; 392: 650-661.
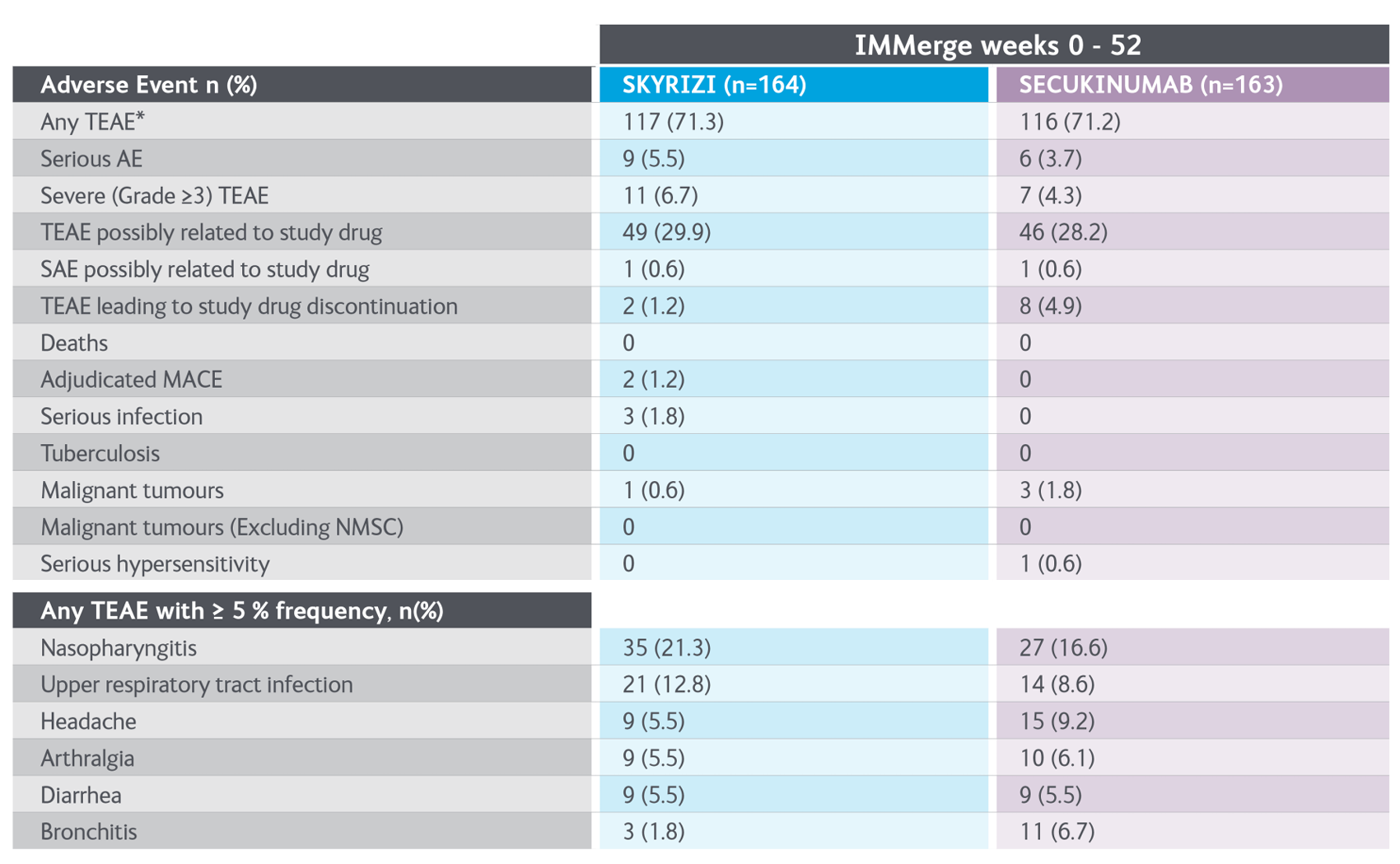
- Warren RB, et al. Risankizumab vs Secukinumab in Patients with Moderate-to-Severe Plaque Psoriasis: A Phase 3 Trial, Presented at AAD 2020.
- SKYRIZI: Summary of Product Characteristics.
- Strober B, et al. Poster 1714, presented at the 28th European Academy of Dermatology and Venereology (EADV) Congress, 9-13 October 2019, Madrid, Spain
- Reich K, et al. Poster 1813, Efficacy and Safety of Risankizumab Compared with Adalimumab in Patients with Moderate-to-Severe Plaque Psoriasis: Results from the Phase 3 IMMvent Trial. 2018.
- Papp KA, et al. Long-Term Safety and Efficacy of Risankizumab for the Treatment of Moderate-to-Severe Plaque Psoriasis: Interim Analysis of the LIMMitless Open-label Extension Trial for up to 6 Years of Follow-up. Presented at EADV, 11–14 October 2023, Berlin, Germany; P2428.
- Kristensen LE, et al. Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 52-Week Results From the KEEPsAKE 1 and KEEPsAKE 2 Trials. Oral presentation EADV 30th Congress 2021 - Anniversary Edition 29 Sept – 2 Oct 2021.
UK-RISN-230324. Date of preparation: February 2024.
Important safety information for SKYRIZI®▼(risankizumab) in Psoriasis and Psoriatic Arthritis¹
SKYRIZI should be initiated and supervised by healthcare professionals experienced in the diagnosis and treatment of Psoriasis and Psoriatic arthritis.
Some patients may not be suitable for SKYRIZI (risankizumab). You are strongly advised to read the prescribing information, which can be found at the top of this webpage and below, and the Summary of Product Characteristics (SmPC) which are available online in the Electronic Medicines Compendium (EMC) from the links below.
SKYRIZI 150 mg solution for injection in pre-filled pen CLICK HERE.
SKYRIZI 150 mg solution for injection in pre-filled syringe CLICK HERE.
SKYRIZI PRESCRIBING INFORMATION CLICK HERE.
SKYRIZI is contraindicated in patients;
- with hypersensitivity to the active substance or to any of the excipients,
- with clinically important active infections (e.g. active tuberculosis).
Cautions (See Prescribing Information and SmPC for full details including screening and monitoring requirements):
It is preferable to avoid the use of SKYRIZI during pregnancy. Women of childbearing potential should use an effective method of contraception during treatment and for at least 21 weeks after treatment. It is unknown whether risankizumab is excreted in human milk. A decision should be made whether to discontinue/abstain from risankizumab therapy, taking into account the benefit of breast-feeding to the child and the benefit of risankizumab therapy to the woman.
Dosing in Psoriasis and Psoriatic Arthritis
The recommended dose of SKYRIZI is 150mg administered by subcutaneous injection at Week 0, Week 4, and every 12 weeks thereafter.
Adverse reactions
For adverse reactions, please refer to the prescribing information and the SKYRIZI summary of product characteristics available online in the Electronic Medicines Compendium via the links above.
Important safety information for HUMIRA® (adalimumab) in Psoriasis and Psoriatic Arthritis²
HUMIRA is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which HUMIRA is indicated.
Psoriatic arthritis: HUMIRA is indicated for the treatment of active and progressive psoriatic arthritis in adults when the response to previous disease-modifying antirheumatic drug therapy has been inadequate. HUMIRA has been shown to reduce the rate of progression of peripheral joint damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease and to improve physical function.
Psoriasis: HUMIRA is indicated for the treatment of moderate-to-severe chronic plaque psoriasis in adult patients who are candidates for systematic therapy
Paediatric plaque psoriasis: HUMIRA is indicated for the treatment of severe chronic plaque psoriasis in children and adolescents from 4 years of age who have had an inadequate response to or are inappropriate candidates for topical therapy and phototherapies.
Some patients may not be suitable for HUMIRA. You are strongly advised to read the prescribing information, which can be found at the top of this webpage and below, and the Summary of Product Characteristics (SmPC) which are available online in the Electronic Medicines Compendium (EMC).
HUMIRA 40 mg solution for injection in pre-filled pen CLICK HERE.
ADALIMUMAB PRESCRIBING INFORMATION CLICK HERE.
References
1. SKYRIZI Summary of Product Characteristics
2. HUMIRA Summary of Product Characteristics