UK-RISN-230338. Date of preparation January 2024.
Find out more about the efficacy of SKYRIZI
Explore the durability of response with SKYRIZI through clinical trials and long-term data in psoriasis and psoriatic arthritis. Start by selecting a study from the menu below.
LONG-TERM DATA
HEAD-TO-HEAD TRIALS
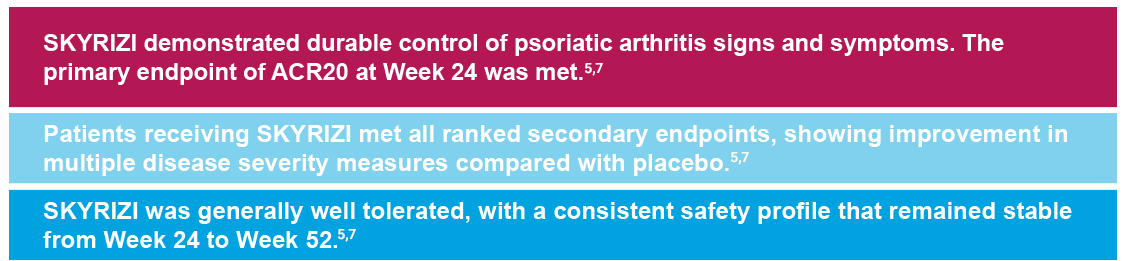
SKYRIZI IN PsA
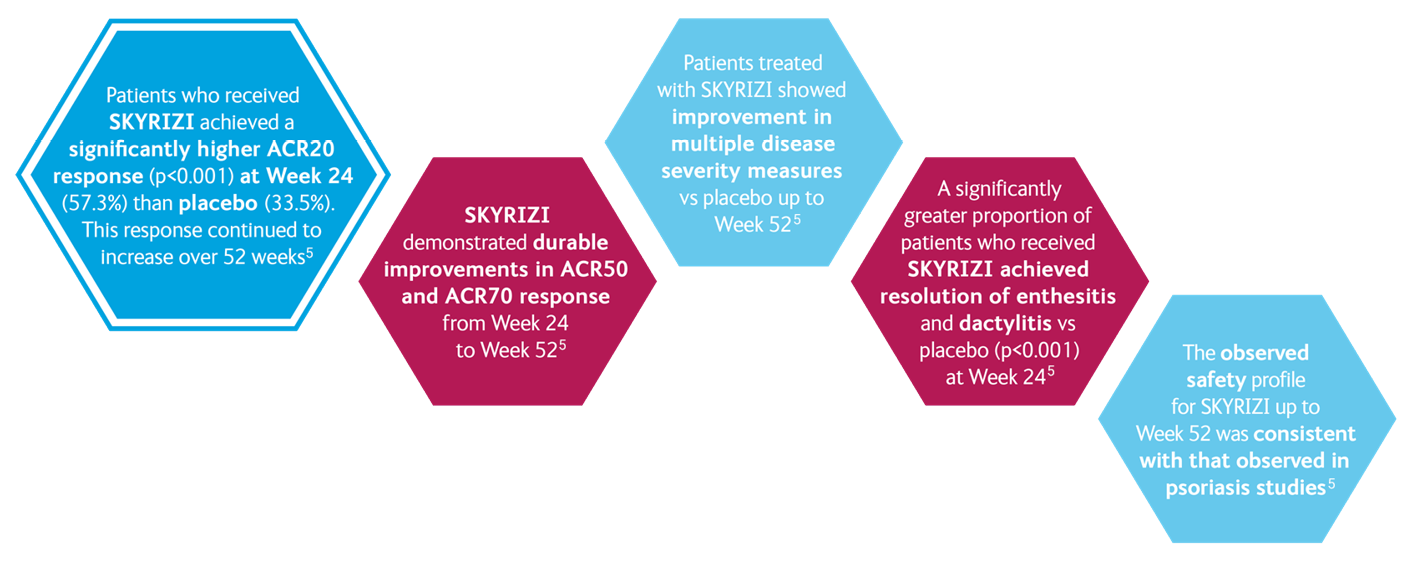
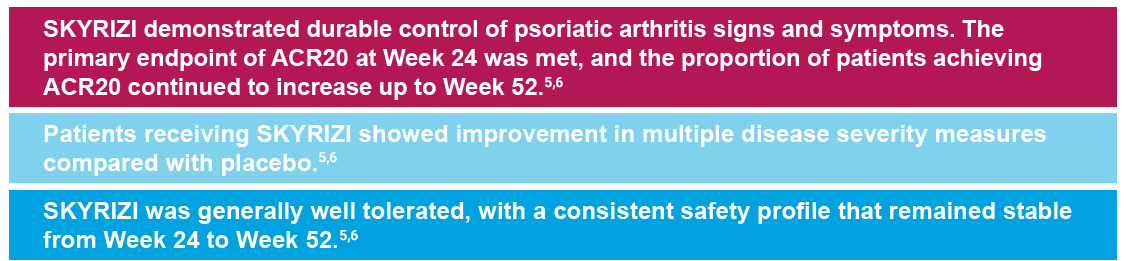
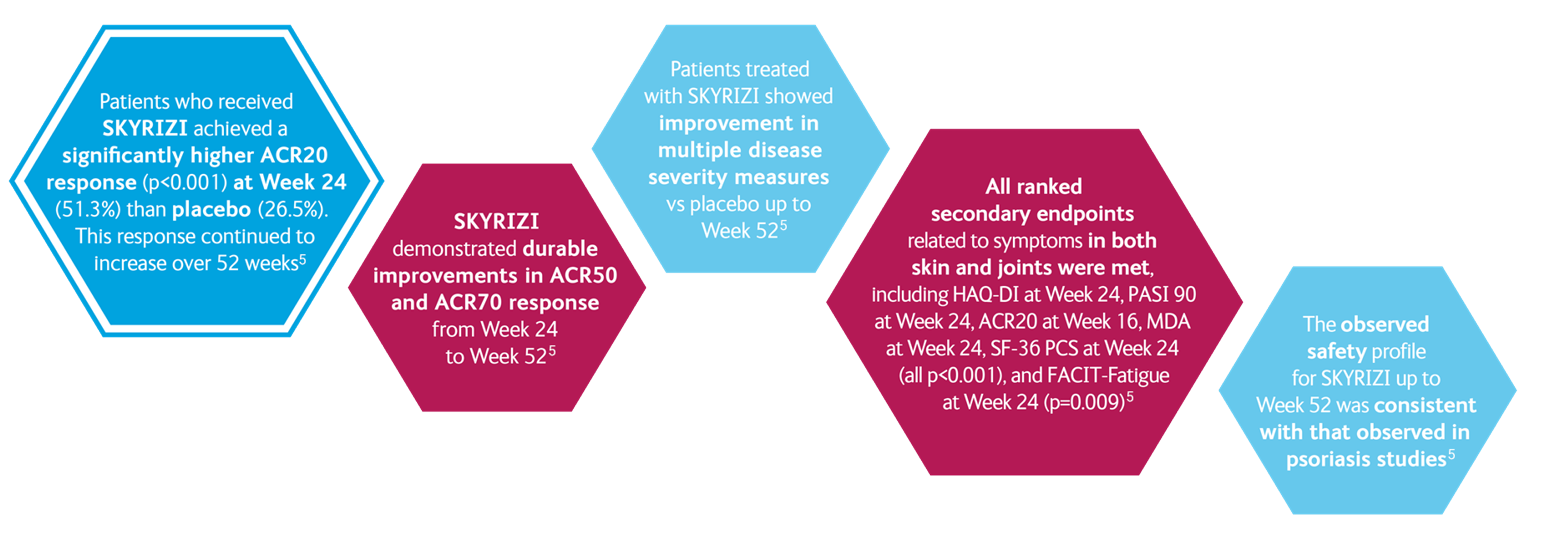
SKYRIZI IN PsA: KEEPsAKE 1 and KEEPsAKE 25-7
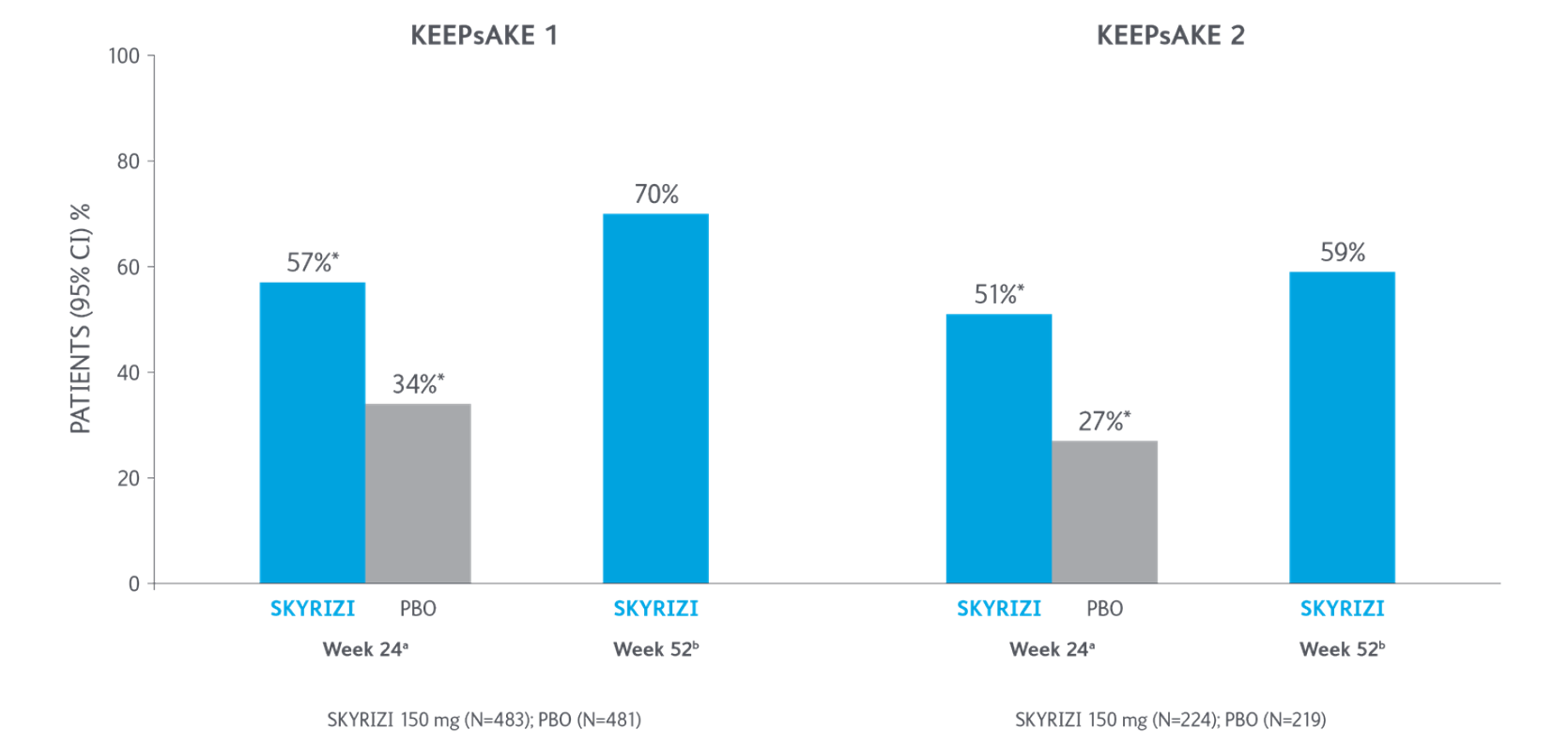
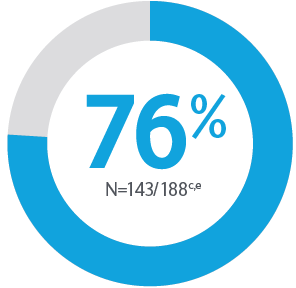
Primary Endpoint ACR20 Response
*P<0.0015-7
Adapted from Kristensen LE, et al. 2021.
aBased on full analysis set, NRI-C was used for missing data.
bBased on full analysis set, NRI (as observed with imputation) was used for missing data.
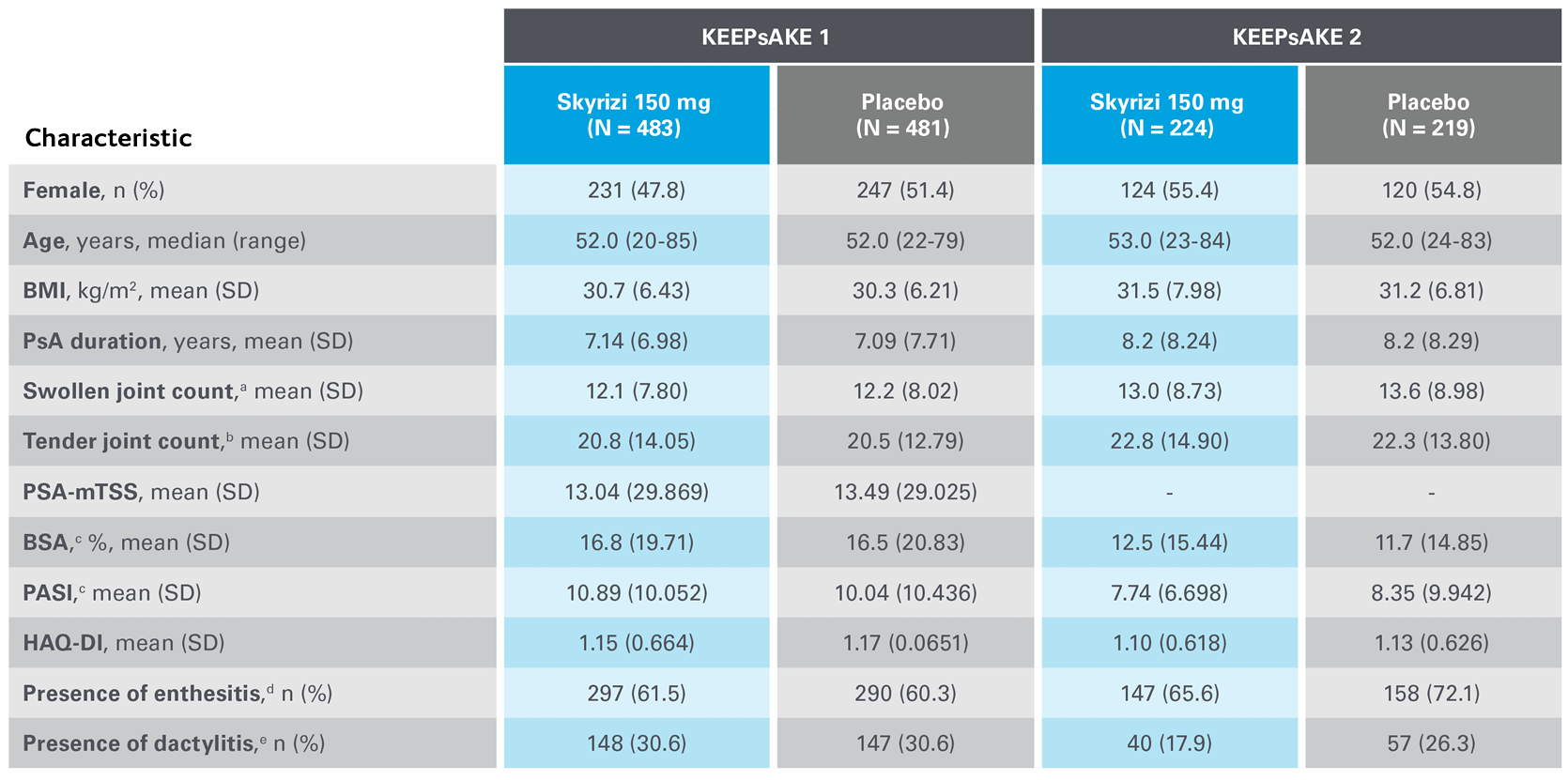
Two randomised, double-blind, placebo-controlled studies assessing the safety and efficacy of 1,407 patients (964 in KEEPsAKE-1 and 443 in KEEPsAKE-2) ≥18 years old with active PsA.
ACR, American College of Rheumatology scale; CI, confidence interval; NRI, nonresponder imputation; NRI-C, nonresponder imputation incorporating multiple imputation to handle missingdata due to COVID-19; PBO, placebo; PsA, psoriatic arthritis.
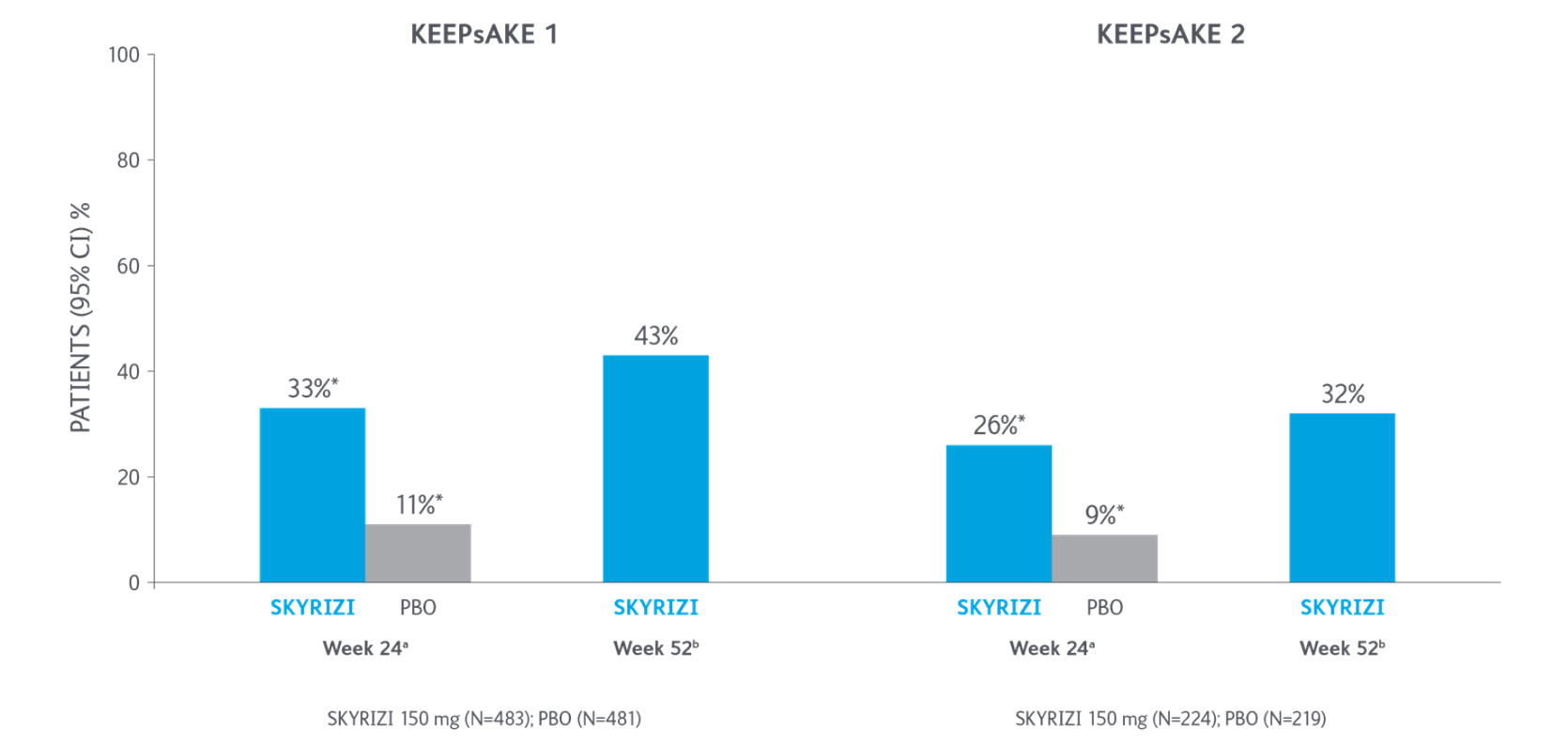
ACR50 Response Over Time
*P<0.0015-7
Adapted from Kristensen LE, et al. 2021.
aBased on full analysis set, NRI-C was used for missing data.
bBased on full analysis set, NRI (as observed with imputation) was used for missing data.
Two randomized, double-blind, placebo-controlled studies assessing the safety and efficacy of 1,407 patients (964 in KEEPsAKE-1 and 443 in KEEPsAKE-2) ≥18 years old with active PsA.
ACR, American College of Rheumatology scale; CI, confidence interval; NRI, nonresponder imputation; NRI-C, nonresponder imputation incorporating multiple imputation to handle missingdata due to COVID-19; PBO, placebo; PsA, psoriatic arthritis.
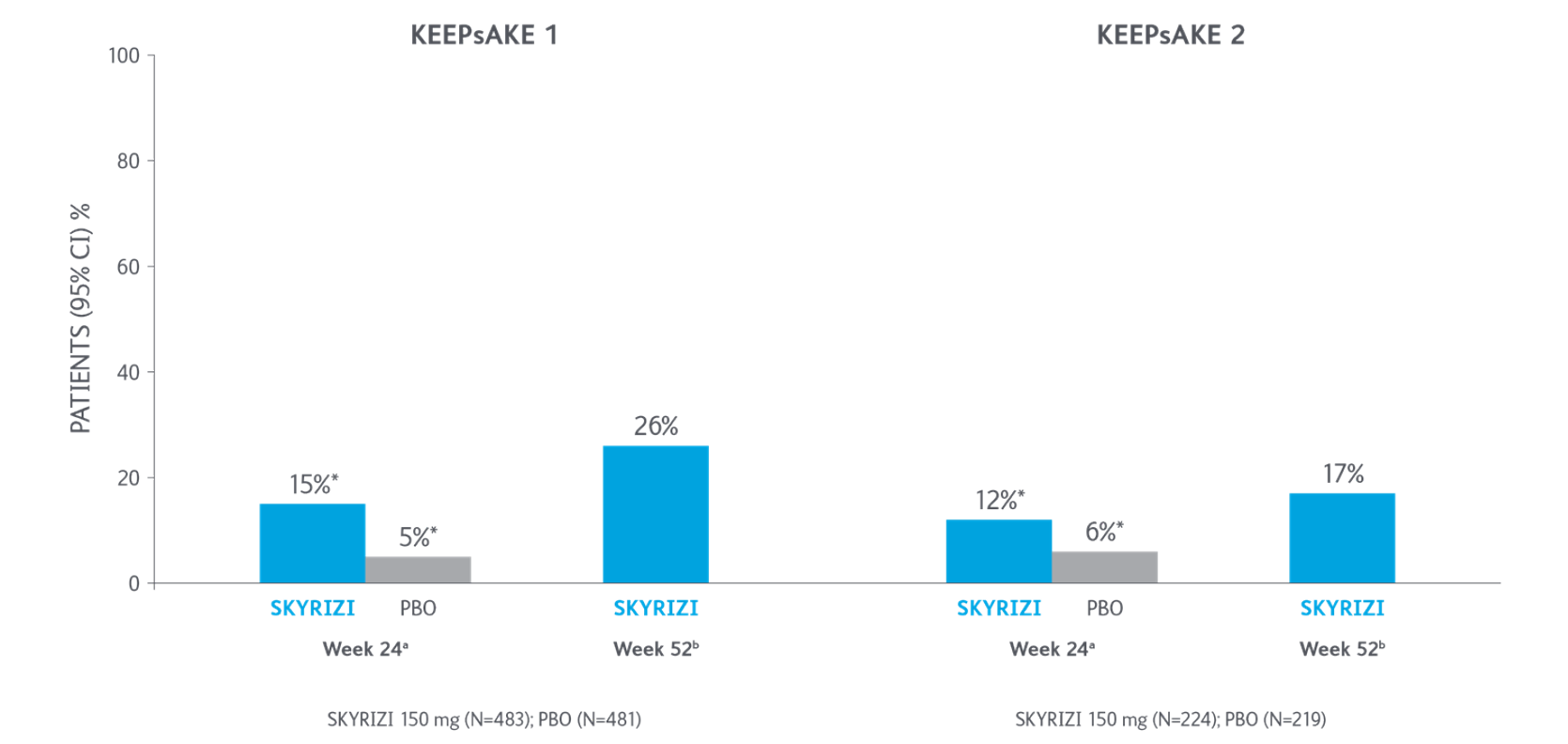
ACR70 Response Over Time
*P<0.0015-7
Adapted from Kristensen LE, et al. 2021.
aBased on full analysis set, NRI-C was used for missing data.
bBased on full analysis set, NRI (as observed with imputation) was used for missing data.
Two randomized, double-blind, placebo-controlled studies assessing the safety and efficacy of 1,407 patients (964 in KEEPsAKE-1 and 443 in KEEPsAKE-2) ≥18 years old with active PsA.
ACR, American College of Rheumatology scale; CI, confidence interval; NRI, nonresponder imputation; NRI-C, nonresponder imputation incorporating multiple imputation to handle missingdata due to COVID-19; PBO, placebo; PsA, psoriatic arthritis.
Two randomised, double-blind, placebo-controlled studies assessing the safety and efficacy of 1,407 patients (964 in KEEPsAKE-1 and 443 in KEEPsAKE-2) ≥18 years old with active PsA
SKYRIZI is dosed 150 mg at Week 0, Week 4, and every 12 weeks thereafter. At week 24 when the placebo cohort were switched over to SKYRIZI, loading doses were not administered and patients were immediately switched to 12 weekly dosing.
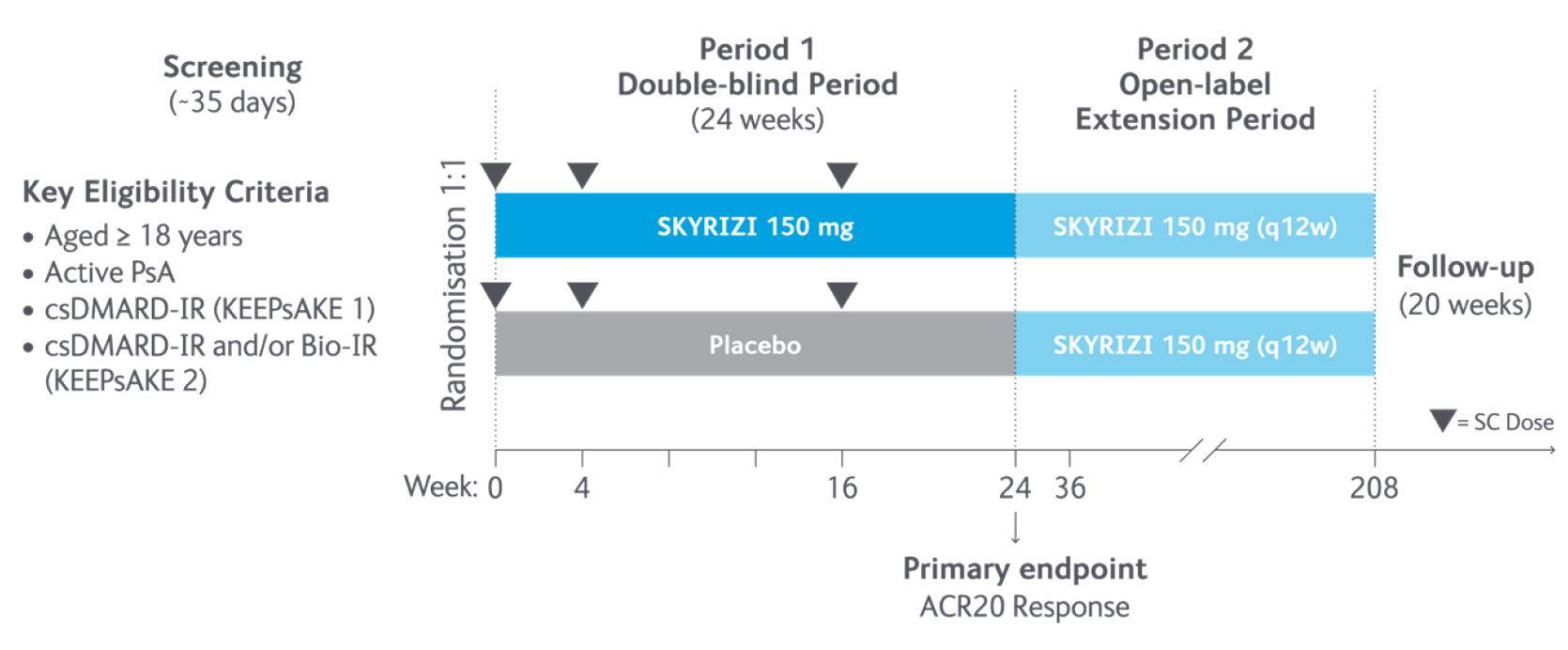
Study design5
In the two randomised, double-blind, placebo-controlled KEEPsAKE-1 and KEEPsAKE-2 studies, patients had a diagnosis of PsA ≥6 months based on Classification Criteria for Psoriatic Arthritis (CASPAR), a median duration of PsA of 4.9 years at baseline, ≥5 tender joints and ≥5 swollen joints, and active plaque psoriasis or nail psoriasis at baseline. 55.9% of subjects had ≥3% body surface area with active plaque psoriasis. 63.4% and 27.9% of subjects had enthesitis and dactylitis, respectively.
In KEEPsAKE-1, all subjects had a previous inadequate response or intolerance to nonbiologic DMARD therapy and were biologic naïve.
In KEEPsAKE-2, 53.5% of subjects had a previous inadequate response or intolerance to nonbiologic DMARD therapy and 46.5% of subjects had a previous inadequate response or intolerance to biologic therapy.
In both studies, subjects were randomised to receive SKYRIZI 150 mg or placebo at Weeks 0, 4, and 16. Starting from Week 28, all subjects received SKYRIZI every 12 weeks. Both studies include a long-term extension for up to an additional 204 weeks. 59.6% of subjects from both studies were receiving concomitant MTX, 11.6% were receiving concomitant nonbiologic DMARDs other than MTX, and 28.9% were receiving SKYRIZI monotherapy. SKYRIZI was dosed 150 mg at Week 0, Week 4, and every 12 weeks thereafter.
Dosing of ustekinumab was 90mg for patients weighing over 100kg.
ACR20, ≥20% improvement in American College of Rheumatology score; CASPAR, Classification Criteria for Psoriatic Arthritis; csDMARD, conventional synthetic disease-modifying antirheumatic drug; MTX, methotrexate; PsA, psoriatic arthritis; q12w, every 12 weeks; SC, subcutaneous.
KEEPsAKE 15,8
- ACR 20 at week 24
KEEPsAKE 25,9
- ACR 20 at week 24
ACR, American College of Rheumatology score.
KEEPsAKE 15,8
- Change in HAQ-DI at week 24
- PASI 90 at week 24*
- ACR20 at week 16
- MDA at week 24
- Change in mNAPSI at week 24
- Change in PGA-F at week 24
- Resolution of Enthesitis†
- Resolution of Dactylitis‡
- Change in PsA-mTSS at week 24
- Change in SF-36 PCS at week 24
- Change in FACIT-Fatigue at week 24
- ACR50 at week 24
- ACR70 at week 24
KEEPsAKE 25,9
- Change in HAQ-DI at week 24
- PASI 90 at week 24*
- ACR20 at week 16
- MDA at week 24
- Change in SF-36 PCS at week 24
- Change in FACIT-Fatigue at week 24
- ACR50 at week 24
- ACR70 at week 24
- Resolution of Enthesitis†
- Resolution of Dactylitis‡
*Among patients with ≥ 3% BSA affected by psoriasis at baseline.
†LEI used to assess the presence or absence of enthesitis.
‡LDI used to assess the presence or absence of dactylitis.
ACR, American College of Rheumatology score; BSA, body surface area; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; PASI, Psoriasis Area and Severity Index; PGA-F, Physician’s Global Assessment of Fingernail Psoriasis; MDA, minimal disease activity; mNAPSI, modified Nail Psoriasis Severity Index; mTSS, medial tibial stress syndrome; SF-36 PCS, 36-Item Short Form Health Survey Physical Component Summary; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy Fatigue Questionnaire.
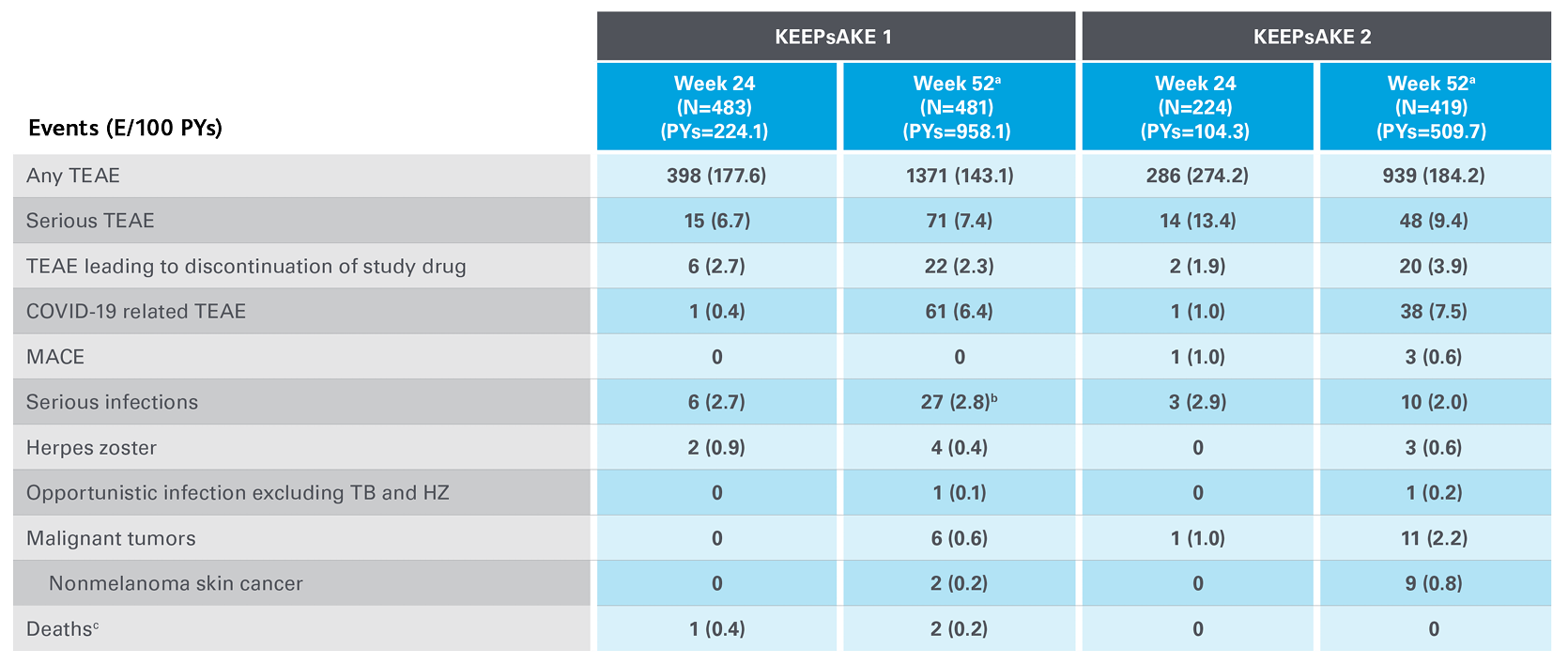
Safety Profile in Psoriatic Arthritis
Safety events from KEEPsAKE 1 and KEEPsAKE 2 through to week 527
Adapted from Kristensen LE, et al. 2021.
aSafety reported through data cutoff date (19 April 2021), which includes data through week 52. Data are from any RZB 150-mg group that includes all patients who received RZB 150 mg, including those who started on RZB 150 mg at randomisation and who switched from placebo to RZB 150 mg after week 24.
b10 of the 27 events were cases of COVID-19.
cAn 81-year-old male patient randomised to RZB died of urosepsis on day 96, and a 41-year-old male patient randomised to RZB experienced sudden death on day 502.
E, events; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer; PY, patient-years; RZB, risankizumab; TB, tuberculosis; TEAE, treatment-emergent adverse events.
Featured content
UK-RISN-230321. Date of preparation February 2024.
UK-RISN-230320. Date of preparation: February 2024.
Important safety information for SKYRIZI®▼(risankizumab) in Psoriasis and Psoriatic Arthritis¹
SKYRIZI should be initiated and supervised by healthcare professionals experienced in the diagnosis and treatment of Psoriasis and Psoriatic arthritis.
Some patients may not be suitable for SKYRIZI (risankizumab). You are strongly advised to read the prescribing information, which can be found at the top of this webpage and below, and the Summary of Product Characteristics (SmPC) which are available online in the Electronic Medicines Compendium (EMC) from the links below.
SKYRIZI 150 mg solution for injection in pre-filled pen CLICK HERE.
SKYRIZI 150 mg solution for injection in pre-filled syringe CLICK HERE.
SKYRIZI PRESCRIBING INFORMATION CLICK HERE.
SKYRIZI is contraindicated in patients;
- with hypersensitivity to the active substance or to any of the excipients,
- with clinically important active infections (e.g. active tuberculosis).
Cautions (See Prescribing Information and SmPC for full details including screening and monitoring requirements):
It is preferable to avoid the use of SKYRIZI during pregnancy. Women of childbearing potential should use an effective method of contraception during treatment and for at least 21 weeks after treatment. It is unknown whether risankizumab is excreted in human milk. A decision should be made whether to discontinue/abstain from risankizumab therapy, taking into account the benefit of breast-feeding to the child and the benefit of risankizumab therapy to the woman.
Dosing in Psoriasis and Psoriatic Arthritis
The recommended dose of SKYRIZI is 150mg administered by subcutaneous injection at Week 0, Week 4, and every 12 weeks thereafter.
Adverse reactions
For adverse reactions, please refer to the prescribing information and the SKYRIZI summary of product characteristics available online in the Electronic Medicines Compendium via the links above.
Important safety information for HUMIRA® (adalimumab) in Psoriasis and Psoriatic Arthritis²
HUMIRA is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which HUMIRA is indicated.
Psoriatic arthritis: HUMIRA is indicated for the treatment of active and progressive psoriatic arthritis in adults when the response to previous disease-modifying antirheumatic drug therapy has been inadequate. HUMIRA has been shown to reduce the rate of progression of peripheral joint damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease and to improve physical function.
Psoriasis: HUMIRA is indicated for the treatment of moderate-to-severe chronic plaque psoriasis in adult patients who are candidates for systematic therapy
Paediatric plaque psoriasis: HUMIRA is indicated for the treatment of severe chronic plaque psoriasis in children and adolescents from 4 years of age who have had an inadequate response to or are inappropriate candidates for topical therapy and phototherapies.
Some patients may not be suitable for HUMIRA. You are strongly advised to read the prescribing information, which can be found at the top of this webpage and below, and the Summary of Product Characteristics (SmPC) which are available online in the Electronic Medicines Compendium (EMC).
HUMIRA 40 mg solution for injection in pre-filled pen CLICK HERE.
ADALIMUMAB PRESCRIBING INFORMATION CLICK HERE.
References
1. SKYRIZI Summary of Product Characteristics
2. HUMIRA Summary of Product Characteristics